Summary
Introduction Durvalumab has been shown to confer a survival benefit after definitive chemoradiotherapy in the patients with locally advanced non-small cell lung cancer, but no studies have attempted to identify risk factors for pneumonitis after durvalumab therapy. The purpose of this study was to investigate associations between clinical and radiation dose-volume factors, and the severity of pneumonitis. Methods We retrospectively assessed the cases of 30 patients who had been started on durvalumab therapy between July 2018 and February 2019. In this study we evaluated the percentage of lung volume receiving radiation dose in excess of 20 Gy (V20) as radiation dose-volume factor. We compared V20 and some baseline factors between a grade 0 or 1 (Gr 0/1) pneumonitis group and a grade 2 or more (≥Gr 2) pneumonitis group, and we performed a logistic regression analysis to establish the associations between variables and ≥ Gr 2 pneumonitis. Results Pneumonitis had developed in 22 patients (73.3%): Gr 1/2/3–5 in 8 (26.7%)/14 (46.7%) /0 (0%), respectively. The difference in V20 between the Gr 0/1 group and Gr 2 group (median: 20.5% vs. 23.5%, p = 0.505) was not statistically significant, and thus V20 was not a risk factor for Gr 2 pneumonitis (odds ratio: 1.047, p = 0.303). None of the clinical factors, including sex, age, smoking history, presence of baseline pneumonitis, type of radiation therapy, location of lesion and facility, were risk factors. Conclusions Our study suggest that the severity of pneumonitis after durvalumab is unrelated to V20 or any of the clinical factors assessed in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Definitive concurrent chemoradiotherapy (CRT) is a standard treatment for unresectable locally advanced non-small cell lung carcinoma (NSCLC). Preclinical studies have shown that radiation therapy synergistically enhances the antitumor effects of immunotherapy, such as with immune checkpoint inhibitors (ICIs), by increasing tumor infiltration and upregulating PD-L1 expression [1, 2]. In addition to its synergistic effect, previous radiotherapy has been reported to activate the antitumor immune response by causing cell death and releasing neoantigen from these dead tumor cells [3, 4]. Durvalumab is an ICI that selectively binds to PD-L1 with high affinity [5]. Durvalumab has been shown to confer a survival benefit after definitive CRT in patients with locally advanced NSCLC in the PACIFIC trial [6, 7].
CRT sometimes causes radiation pneumonitis. A meta-analysis reported that the incidence of radiation pneumonitis is 5%–50% rate, and that the mortality rate is 1%–2% [8], but it is unknown whether combining ICI therapy with CRT increases the risk of pneumonitis. The incidence of any grade (Gr) pneumonitis in the durvalumab arm of the PACIFIC trial was 33.9%, and the incidence of Gr 3/4 pneumonitis was 3.4%. but the incidences in the Japanese subgroup were higher: any Gr, 73.6% and Gr 3/4, 5.6%.
Lung V20, the percentage of lung volume receiving radiation dose in excess of 20 Gy, is the most common radiation dose-volume factor for radiation pneumonitis [9, 10]. Other risk factors for RP, including age, concurrent CRT regimen, location of the primary tumor lesion, and pre-existing lung disease, have also been reported [11,12,13], and female and never-smoker may be risk factors, but whether they are has been a matter of controversy [14, 15]. However, no studies have investigated whether any of these factors, including V20, are related to the development of pneumonitis after durvalumab administration following CRT, and there was no information about factors related to pneumonitis in the PACIFIC trial. We hypothesized that Lung V20 and other baseline clinical factors have an impact on the development of pneumonitis after durvalumab administration. The purpose of this study was to assess these factors for associations with the grade of pneumonitis.
Methods
Forty-one patients with stage III NSCLC received definitive CRT at our institution between April 2018 and December 2018, and 25 of these patients, with no progression after CRT, had been started on durvalumab between July 2018 and February 2019. Besides, another five patients received CRT at another institution and received durvalumab at our institution (Fig. 1). We retrospectively reviewed the data of these 30 patients. We decided whether CRT and durvalumab were indicated in each patient at a multidisciplinary conference. Durvalumab was administered in a dose of 10 mg/kg every 2 weeks until disease progression or intolerance, for up to 12 months. Twenty-one patients were treated with three-dimensional conformal radiation, and two patients were treated with intensity modulated radiation therapy (IMRT). Seven patients were treated with proton beam therapy (PBT) after approval was obtained at a multidisciplinary conference. We selected to use IMRT/PBT when the irradiation doses to normal tissue, such as lung and spinal cord, were likely to exceed acceptable range. The total radiation dose in all patients was 60 Gy or 64 Gy.
We compared V20 and baseline clinical factors between two groups, a Gr 0/1 pneumonitis group and ≥ Gr 2 pneumonitis group, and performed a univariate and multivariate analysis of risk factors for the pneumonitis. We diagnosed pneumonitis on the basis of the CT scan or X-ray evidence when a patient had a fever or cough, and, in the absence of symptoms, by chance at follow-up. Patients with an apparent pulmonary infection or heart failure were excluded. The grade of pneumonitis was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. The patients with Gr 1 pneumonitis at baseline who did not experience an exacerbation after durvalumab administration were classified as Gr 0, and those with an exacerbation, as Gr 2. These criteria were consistent with those used in the Pacific trial.
Candidate variables for associations with Gr 2 pneumonitis included sex, age, smoking history, presence of Gr 1 pneumonitis at baseline, type of radiation therapy (X-ray or proton beam), location of the lesion (upper lobe or lower lobe), and facility where CRT was performed (our institution or other institution).
Statistical analysis
Comparisons between categorical variables were performed using Fisher’s exact test, and comparisons between continuous variables (V20) were performed using Wilcoxon test. A multivariate logistic regression analysis was used to establish associations between these variables and ≥ Gr 2 pneumonitis. A p value of <0.05 was considered statistically significant in all analyses.
Results
The patients’ characteristics are summarized in Table 1. Their median age was 68 years (range: 47–78 years). They included four patients who had received CRT for mediastinal lymph node (#3a, #4 L, #4R, #7) recurrence, and all four patients were in the upper lobe lesion group. The concurrent chemotherapy regimen was cisplatin + S-1 in 13 patients, carboplatin + paclitaxel in 7 patients, cisplatin + vinorelbine in 4 patients, daily carboplatin in 4 patients, and carboplatin + nab-paclitaxel in 7 patients. The PD-L1 tumor proportion score (TPS) with the 22C3 assay was calculated in 14 patients; 5 of them had a PD-L1 TPS <1%, and 4 of them had a PD-L1 TPS ≥50%.
The median follow-up time was 7.7 months (range: 2.9–10.4 months). Six of the 30 patients had Gr 1 pneumonitis at baseline, and the pneumonitis exacerbated to Gr 2 in 2 of them. Of the 24 patients with no pneumonitis at baseline, 20 patients developed pneumonitis after durvalumab administration: Gr 1 in 8 patients and Gr 2 pneumonitis in 14 patients, including 2 patients with pneumonitis at baseline. No patients developed Gr 3–5 pneumonitis. Thus, pneumonitis developed in 22 patients (73.3%), and that of each grade was as follows: Gr 1/Gr 2/Gr 3–5 in 8 (26.7%)/14 (46.7%)/0 (0%), respectively. The pneumonitis developed at a median interval of 2.2 months (range: 0.6–3.4 months) after the completion of radiotherapy and a median of 1.4 months (range: 0.5–2.1 months) after the final dose of durvalumab. No pneumonitis developed beyond the radiation field.
Of the 14 patients with Gr 2 pneumonitis, 6 patients (27.3%) required prednisolone (PSL) therapy because their pneumonitis did not improve after durvalumab suspension, and all 6 patients recovered rapidly in response to PSL. In 10 patients (Gr 1: 3, Gr 2: 7), durvalumab could be re-administered after their pneumonitis was resolved. There were no exacerbations of pneumonitis during a median interval of 5.9 months (range: 1.1–7.5 months) after re-administration.
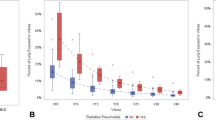
The relation between the grade of pneumonitis and V20 is shown in Fig. 2. The results of the univariate analysis did not show a significant difference in V20 between the G 0/1 group and G2 group (median: 20.5% vs. 23.5%, p = 0.505, Wilcoxon test) (Table 1), and thus V20 could not be a risk factor for Gr 2 pneumonitis (odds ratio: 1.047, p = 0.303) (Table 2). Even when the cut-off value was set to 26% as in previous study [10], V20 could not be a risk factor (odds ratio: 2.25, p = 0.305). The results also showed that none of the other factors could be risk factors for Gr 2 pneumonitis (Table 2), and, thus, a multivariate analysis could not be performed.
Relationship between grade of pneumonitis and V20. The cases are arranged in ascending order of their V20 values (plotted on the horizontal axis). Blue/green/red bars represent Gr 0/1/2 pneumonitis, respectively. “*” indicates treatment with proton beam therapy, and “<” indicates a case with Gr1 pneumonitis at baseline
Discussion
We report the relation of V20 and some baseline clinical factors to the grade of pneumonitis after durvalumab administration following CRT. Although radiation pneumonitis has reported to be associated with V20 and some clinical factors in the previous studies, the results of our study did not show a statistically significant association between any of the factors, including V20, and pneumonitis after durvalumab administration. To our knowledge, this is the first study to attempt to identify risk factors for pneumonitis after durvalumab administration.
The incidence of pneumonitis in this study was almost the same as in the Japanese subgroup of the PACIFIC trial. However, none of the patients in our study developed severe pneumonitis (Gr 3–5), and only a few patients developed severe pneumonitis in the PACIFIC trial. Since the Japanese clinical guideline for lung cancer state that patients with a V20 ≥ 35% are highly likely to develop radiation pneumonitis, we avoid irradiating such patients in consultation with radiation oncologists. This patient selection perhaps prevented the development of severe pneumonitis. Interestingly, a smaller percentage of patients with pneumonitis required PSL administration in our study (27.3%) than in the PACIFIC trial (62.1%), because the protocol of the PACIFIC trial recommended that all patients with Gr 2 pneumonitis be started on systemic steroid therapy promptly [6]. On the other hand, 7 (50%) of the 14 patients with Gr 2 pneumonitis in our study were cured by durvalumab suspension alone. This may be explained by the fact that we monitored the course of pneumonitis without PSL therapy for one to two weeks in accordance with the strategy for radiation pneumonitis [16]. Another reason might be that no pneumonitis developed beyond the radiation field because of proper radiation treatment planning. Not all patients with Gr 2 pneumonitis may require PSL therapy, but we could not identify the clinical differences between the group of patients who needed PSL and the group that did not, because the number of subjects was small.
The results of this study did not show any statistically significant associations between V20 and the grade of pneumonitis after durvalumab administration. As stated above, there were no patients with a V20 ≥ 35% in this study. Tsujino K et al. [10] reported finding that the 6-month cumulative incidence of Gr 2 radiation pneumonitis increased with V20 even when V20 was less than 35%. The pneumonitis after durvalumab administration is induced by the ICI as well as by the radiation, so factors other than dosimetric factors such as V20 may be more important. Radiation therapy induces tumor cell death, which leads to an increase in tumor-infiltrating lymphocytes (TILs) in the microenvironment and to upregulation of PD-L1 on the surface of the tumor [2]. These factors may be responsible for the differences in immune-related adverse events across patients or tumor types [17]. Similarly, biomarkers such as TILs and PD-L1 are likely to influence the severity of the pneumonitis after durvalumab administration.
The pneumonitis that develops after durvalumab is different from radiation pneumonitis in terms of radiological features. Although it is difficult to distinguish between the two forms of pneumonitis, ICI-related pneumonitis has been reported to exhibit some specific radiological features, such as ground glass opacities (GGOs), a cryptogenic organizing pneumonia-like appearance, and interstitial pneumonia pattern [18]. Baba T et al. [19] reported that GGOs around the tumor (peritumoral infiltration) were a characteristic finding in pneumonitis induced by nivolumab.
ICIs, including durvalumab, have caused pneumonitis in the rate of 2.7%–5% in some recent reports [20,21,22]. Few studies have identified baseline clinical factors associated with the ICI-induced pneumonitis. Naidoo J et al. [22] reported significant associations between both current/former smokers and underlying lung conditions and the worsening of pneumonitis by ICI monotherapy or ICI combination therapy. However, smoking history and baseline pneumonitis were not found to be risk factors in our study. Unfortunately, we were unable to identify any clinical factors associated with the pneumonitis after durvalumab administration following CRT. The involvement of both radiation and ICIs may complicate the detection of the risk factors for this pneumonitis.
This study had two limitations. First, the subjects were a small number of patients at a single institution. Second, the follow-up period was too short to draw a conclusion. Although all of the cases of pneumonitis in this study developed within 3 months after the final durvalumab dose, pneumonitis is likely to develop after follow-up time. Further study with a longer follow-up time will be needed to confirm our results.
In conclusion, the results of our study suggest that the severity of pneumonitis after durvalumab administration are unrelated to V20 or any other clinical factors that we assessed in this study. Further investigations are warranted, for finding out other factors that affect developing the pneumonitis.
Change history
26 June 2020
Corrections are needed to the original version of this article. In Table 2, the “Odds ratio” of the variables “Lower lobe vs. Upper lobe” and “≥26 vs. <26” should be 2.250 instead of 2250 and 2.250 instead of 2.25.
References
Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX (2014) Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 124:687–695
Sharabi AB, Lim M, DeWeese TL, Drake CG (2015) Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 16:e498–e509
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, Garon EB, Lee P (2017) Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 18:895–903
Demaria S, Golden EB, Formenti SC (2015) Role of local radiation therapy in Cancer immunotherapy. JAMA Oncol 1:1325–1332
Stewart R, Morrow M, Hammond SA, Mulgrew K, Marcus D, Poon E, Watkins A, Mullins S, Chodorge M, Andrews J, Bannister D, Dick E, Crawford N, Parmentier J, Alimzhanov M, Babcook JS, Foltz IN, Buchanan A, Bedian V, Wilkinson RW, McCourt M (2015) Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res 3:1052–1062
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M, PACIFIC Investigators (2017) Durvalumab after Chemoradiotherapy in stage III non-small-cell lung Cancer. N Engl J Med 377:1919–1929
Antonia SJ, Villegas A, Daniel D et al (2018) Overall survival with Durvalumab after Chemoradiotherapy in stage III NSCLC. N Engl J Med 379:2342–2350
Palma DA, Senan S, Tsujino K et al (2013) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85:444–450
Graham MV, Purdy JA, Emami B et al (1999) Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phy 45:323–329
Tsujino K, Hirota S, Endo M et al (2003) Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys 55:110–115
Marks LB, Bentzen SM, Deasy JO et al (2010) Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phy 76:S70–S76
Rodrigues G, Lock M, D'Souza D, Yu E, van Dyk J (2004) Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer--a systematic review. Radiother Oncol 71:127–138
Mehta V (2005) Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys 63:5–24
Vogelius IR, Bentzen SM (2012) A literature-based meta-analysis of clinical risk factors for development of radiation induced pneumonitis. Acta Oncol 51:975–983
Kong FM, Wang S (2012) Non-dosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol 25:100–109
Sekine I, Sumi M, Ito Y et al (2006) Retrospective analysis of steroid therapy for radiation-induced lung injury in lung cancer patients. Radiother Oncol 80:93–97
Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR (2017) Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 28:2377–2385
Jbag H, Carbonnel F, Robert C et al (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol suppl_4(28):119–142
Baba T, Sakai F, Kato T et al (2019) Radiologic features of pneumonitis associated with nivolumab in non-small-cell lung cancer and malignant melanoma. Future Oncol 15:1911–1920
Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, Hatabu H, Ott PA, Armand PF, Hodi FS (2016) PD-1 inhibitor-related pneumonitis in advanced cancer patients: radiographic patterns and clinical course. Clin Cancer Res 22:6051–6060
Delaunay M, Cadranel J, Lusque A et al (2017) Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J 50:1700050
Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD (2017) Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 35:709–717
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
H Inoue declares that he has no conflict of interest. A Ono received honoraria from AstraZeneca. T Kawabata declares that he has no conflict of interest. N mamesaya received honoraria from AstraZeneca. T Kawamura declares that he has no conflict of interest. H Kobayashi declares that he has no conflict of interest. S Omori received honoraria from AstraZeneca. K Wakuda received honoraria from AstraZeneca. H Kenmotsu received honoraria and research funding to our institution from AstraZeneca. T Naito declares that he has no conflict of interest. H Murakami received honoraria and research funding to our institution from AstraZeneca. K Yasui declares that he has no conflict of interest. H Ogawa declares that he has no conflict of interest. T Onoe declares that he has no conflict of interest. M Endo received honoraria from AstraZeneca. H Harada received honoraria from AstraZeneca. T Takahashi received honoraria and research funding to our institution from AstraZeneca.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional Review Board of Shizuoka Cancer Center (reference number: 30-J134–30–1-3) and with the 1964 Helsinki declaration and its later amendments or with comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Inoue, H., Ono, A., Kawabata, T. et al. Clinical and radiation dose-volume factors related to pneumonitis after treatment with radiation and durvalumab in locally advanced non-small cell lung cancer. Invest New Drugs 38, 1612–1617 (2020). https://doi.org/10.1007/s10637-020-00917-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00917-2