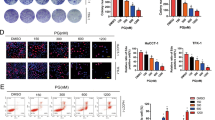
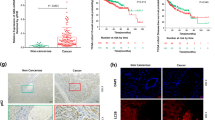
Summary
Among the acquired modifications in cancer cells, changes in lysosomal phenotype and functions are well described, making lysosomes a potential target for novel therapies. Some weak base lipophilic drugs have a particular affinity towards lysosomes, taking benefits from lysosomal trapping to exert anticancer activity. Here, we have developed a new lysosomotropic small molecule, GNS561, and assessed its activity in multiple in vitro intrahepatic cholangiocarcinoma models (HuCCT1 and RBE cell lines and patient-derived cells) and in a chicken chorioallantoic membrane xenograft model. GNS561 significantly reduced cell viability in two intrahepatic cholangiocarcinoma cell lines (IC50 of 1.5 ± 0.2 μM in HuCCT1 and IC50 of 1.7 ± 0.1 μM in RBE cells) and induced apoptosis as measured by caspases activation. We confirmed that GNS561-mediated cell death was related to its lysosomotropic properties. GNS561 induced lysosomal dysregulation as proven by inhibition of late-stage autophagy and induction of a dose-dependent build-up of enlarged lysosomes. In patient-derived cells, GNS561 was more potent than cisplatin and gemcitabine in 2/5 and 1/5 of the patient-derived cells models, respectively. Moreover, in these models, GNS561 was potent in models with low sensitivity to gemcitabine. GNS561 was also efficient in vivo against a human intrahepatic cholangiocarcinoma cell line in a chicken chorioallantoic membrane xenograft model, with a good tolerance at doses high enough to induce an antitumor effect in this model. In summary, GNS561 is a new lysosomotropic agent, with an anticancer activity against intrahepatic cholangiocarcinoma. Further investigations are currently ongoing to fully elucidate its mechanism of action.




Similar content being viewed by others
References
World Health Organization (2018) Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed December 18, 2018
Kirstein MM, Vogel A (2016) Epidemiology and risk factors of cholangiocarcinoma. Visc Med 32(6):395–400. https://doi.org/10.1159/000453013
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, Investigators ABCT (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362(14):1273–1281. https://doi.org/10.1056/NEJMoa0908721
El-Serag HB, Engels EA, Landgren O, Chiao E, Henderson L, Amaratunge HC, Giordano TP (2009) Risk of hepatobiliary and pancreatic cancers after hepatitis C virus infection: a population-based study of U.S. veterans. Hepatology 49(1):116–123. https://doi.org/10.1002/hep.22606
Ariizumi S, Yamamoto M (2015) Intrahepatic cholangiocarcinoma and cholangiolocellular carcinoma in cirrhosis and chronic viral hepatitis. Surg Today 45(6):682–687. https://doi.org/10.1007/s00595-014-1031-0
Razumilava N, Gores GJ (2014) Cholangiocarcinoma. Lancet 383(9935):2168–2179. https://doi.org/10.1016/S0140-6736(13)61903-0
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289. https://doi.org/10.1016/j.jhep.2014.01.021
Lamarca A, Hubner RA, David Ryder W, Valle JW (2014) Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 25(12):2328–2338. https://doi.org/10.1093/annonc/mdu162
Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H, Feld R, Moore M, Knox JJ (2013) Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer 49(2):329–335. https://doi.org/10.1016/j.ejca.2012.08.003
Mahipal A, Kommalapati A, Tella SH, Lim A, Kim R (2018) Novel targeted treatment options for advanced cholangiocarcinoma. Expert Opin Investig Drugs 27(9):709–720. https://doi.org/10.1080/13543784.2018.1512581
Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, Hama N, Hosoda F, Urushidate T, Ohashi S, Hiraoka N, Ojima H, Shimada K, Okusaka T, Kosuge T, Miyagawa S, Shibata T (2015) Genomic spectra of biliary tract cancer. Nat Genet 47(9):1003–1010. https://doi.org/10.1038/ng.3375
Kallunki T, Olsen OD, Jaattela M (2013) Cancer-associated lysosomal changes: friends or foes? Oncogene 32(16):1995–2004. https://doi.org/10.1038/onc.2012.292
Perera RM, Stoykova S, Nicolay BN, Ross KN, Fitamant J, Boukhali M, Lengrand J, Deshpande V, Selig MK, Ferrone CR, Settleman J, Stephanopoulos G, Dyson NJ, Zoncu R, Ramaswamy S, Haas W, Bardeesy N (2015) Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 524(7565):361–365. https://doi.org/10.1038/nature14587
Appelqvist H, Waster P, Kagedal K, Ollinger K (2013) The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol 5(4):214–226. https://doi.org/10.1093/jmcb/mjt022
De Duve C, Pressman BC, Gianetto R, Wattiaux R, Appelmans F (1955) Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J 60(4):604–617
Xu H, Ren D (2015) Lysosomal physiology. Annu Rev Physiol 77:57–80. https://doi.org/10.1146/annurev-physiol-021014-071649
de Duve C (1983) Lysosomes revisited. Eur J Biochem 137(3):391–397
Boyer MJ, Tannock IF (1993) Lysosomes, lysosomal enzymes,. Adv Cancer Res 60:269–291
Kroemer G, Jaattela M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5(11):886–897. https://doi.org/10.1038/nrc1738
Castino R, Demoz M, Isidoro C (2003) Destination 'lysosome': a target organelle for tumour cell killing? J Mol Recognit 16(5):337–348. https://doi.org/10.1002/jmr.643
Hamalisto S, Jaattela M (2016) Lysosomes in cancer-living on the edge (of the cell). Curr Opin Cell Biol 39:69–76. https://doi.org/10.1016/j.ceb.2016.02.009
Davidson SM, Vander Heiden MG (2017) Critical functions of the lysosome in Cancer biology. Annu Rev Pharmacol Toxicol 57:481–507. https://doi.org/10.1146/annurev-pharmtox-010715-103101
Morgan MJ, Fitzwalter BE, Owens CR, Powers RK, Sottnik JL, Gamez G, Costello JC, Theodorescu D, Thorburn A (2018) Metastatic cells are preferentially vulnerable to lysosomal inhibition. Proc Natl Acad Sci U S A 115(36):E8479–E8488. https://doi.org/10.1073/pnas.1706526115
Martinez-Carreres L, Nasrallah A, Fajas L (2017) Cancer: linking powerhouses to suicidal bags. Front Oncol 7:204. https://doi.org/10.3389/fonc.2017.00204
Saftig P, Sandhoff K (2013) Cancer: killing from the inside. Nature 502(7471):312–313. https://doi.org/10.1038/nature12692
Boya P, Kroemer G (2008) Lysosomal membrane permeabilization in cell death. Oncogene 27(50):6434–6451. https://doi.org/10.1038/onc.2008.310
Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM (2003) Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5(6):533–545
Zhitomirsky B, Assaraf YG (2016) Lysosomes as mediators of drug resistance in cancer. Drug Resist Updat 24:23–33. https://doi.org/10.1016/j.drup.2015.11.004
Fehrenbacher N, Gyrd-Hansen M, Poulsen B, Felbor U, Kallunki T, Boes M, Weber E, Leist M, Jaattela M (2004) Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res 64(15):5301–5310. https://doi.org/10.1158/0008-5472.CAN-04-1427
Domagala A, Fidyt K, Bobrowicz M, Stachura J, Szczygiel K, Firczuk M (2018) Typical and atypical inducers of lysosomal cell death: a promising anticancer strategy. Int J Mol Sci 19(8). https://doi.org/10.3390/ijms19082256
Fennelly C, Amaravadi RK (2017) Lysosomal biology in cancer. Methods Mol Biol 1594:293–308. https://doi.org/10.1007/978-1-4939-6934-0_19
Kirkegaard T, Jaattela M (2009) Lysosomal involvement in cell death and cancer. Biochim Biophys Acta 1793(4):746–754. https://doi.org/10.1016/j.bbamcr.2008.09.008
Ono K, Kim SO, Han J (2003) Susceptibility of lysosomes to rupture is a determinant for plasma membrane disruption in tumor necrosis factor alpha-induced cell death. Mol Cell Biol 23(2):665–676
Petersen NH, Olsen OD, Groth-Pedersen L, Ellegaard AM, Bilgin M, Redmer S, Ostenfeld MS, Ulanet D, Dovmark TH, Lonborg A, Vindelov SD, Hanahan D, Arenz C, Ejsing CS, Kirkegaard T, Rohde M, Nylandsted J, Jaattela M (2013) Transformation-associated changes in sphingolipid metabolism sensitize cells to lysosomal cell death induced by inhibitors of acid sphingomyelinase. Cancer Cell 24(3):379–393. https://doi.org/10.1016/j.ccr.2013.08.003
Piao S, Amaravadi RK (2016) Targeting the lysosome in cancer. Ann N Y Acad Sci 1371(1):45–54. https://doi.org/10.1111/nyas.12953
Serrano-Puebla A, Boya P (2018) Lysosomal membrane permeabilization as a cell death mechanism in cancer cells. Biochem Soc Trans 46(2):207–215. https://doi.org/10.1042/BST20170130
Wang F, Gomez-Sintes R, Boya P (2018) Lysosomal membrane permeabilization and cell death. Traffic 19(12):918–931. https://doi.org/10.1111/tra.12613
Halaby R (2015) Role of lysosomes in cancer therapy. Res Rep Biol 6:147–155. https://doi.org/10.2147/RRB.S83999
Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, Tang L, Wang M, Wang Q, Wang HY (2011) Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Investig 91(8):1146–1157. https://doi.org/10.1038/labinvest.2011.97
Nitta T, Sato Y, Ren XS, Harada K, Sasaki M, Hirano S, Nakanuma Y (2014) Autophagy may promote carcinoma cell invasion and correlate with poor prognosis in cholangiocarcinoma. Int J Clin Exp Pathol 7(8):4913–4921
Thongchot S, Yongvanit P, Loilome W, Seubwai W, Phunicom K, Tassaneeyakul W, Pairojkul C, Promkotra W, Techasen A, Namwat N (2014) High expression of HIF-1alpha, BNIP3 and PI3KC3: hypoxia-induced autophagy predicts cholangiocarcinoma survival and metastasis. Asian Pac J Cancer Prev 15(14):5873–5878
Sasaki M, Nitta T, Sato Y, Nakanuma Y (2015) Autophagy may occur at an early stage of cholangiocarcinogenesis via biliary intraepithelial neoplasia. Hum Pathol 46(2):202–209. https://doi.org/10.1016/j.humpath.2014.09.016
Boya P, Gonzalez-Polo RA, Poncet D, Andreau K, Vieira HL, Roumier T, Perfettini JL, Kroemer G (2003) Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene 22(25):3927–3936. https://doi.org/10.1038/sj.onc.1206622
Zhang Y, Liao Z, Zhang LJ, Xiao HT (2015) The utility of chloroquine in cancer therapy. Curr Med Res Opin 31(5):1009–1013. https://doi.org/10.1185/03007995.2015.1025731
Fu W, Li X, Lu X, Zhang L, Li R, Zhang N, Liu S, Yang X, Wang Y, Zhao Y, Meng X, Zhu WG (2017) A novel acridine derivative, LS-1-10 inhibits autophagic degradation and triggers apoptosis in colon cancer cells. Cell Death Dis 8(10):e3086. https://doi.org/10.1038/cddis.2017.498
Xu R, Ji Z, Xu C, Zhu J (2018) The clinical value of using chloroquine or hydroxychloroquine as autophagy inhibitors in the treatment of cancers: a systematic review and meta-analysis. Medicine (Baltimore) 97(46):e12912. https://doi.org/10.1097/MD.0000000000012912
Rebecca VW, Amaravadi RK (2016) Emerging strategies to effectively target autophagy in cancer. Oncogene 35(1):1–11. https://doi.org/10.1038/onc.2015.99
Manic G, Obrist F, Kroemer G, Vitale I, Galluzzi L (2014) Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell Oncol 1(1):e29911. https://doi.org/10.4161/mco.29911
Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V, Agostinis P, Bouche G (2017) Repurposing drugs in oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience 11:781. https://doi.org/10.3332/ecancer.2017.781
Plantone D, Koudriavtseva T (2018) Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig 38(8):653–671. https://doi.org/10.1007/s40261-018-0656-y
Pascolo S (2016) Time to use a dose of chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J Pharmacol 771:139–144. https://doi.org/10.1016/j.ejphar.2015.12.017
Bernstein HN (1991) Ocular safety of hydroxychloroquine. Ann Ophthalmol 23(8):292–296
Prudent R, Vassal-Stermann E, Nguyen CH, Mollaret M, Viallet J, Desroches-Castan A, Martinez A, Barette C, Pillet C, Valdameri G, Soleilhac E, Di Pietro A, Feige JJ, Billaud M, Florent JC, Lafanechere L (2013) Azaindole derivatives are inhibitors of microtubule dynamics, with anti-cancer and anti-angiogenic activities. Br J Pharmacol 168(3):673–685. https://doi.org/10.1111/j.1476-5381.2012.02230.x
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar S, Viallet J, Saleh A, Al Agha H, Eid A, Iratni R (2013) Anti-metastatic and anti-tumor growth effects of Origanum majorana on highly metastatic human breast cancer cells: inhibition of NFkappaB signaling and reduction of nitric oxide production. PLoS One 8(7):e68808. https://doi.org/10.1371/journal.pone.0068808
El Hasasna H, Saleh A, Al Samri H, Athamneh K, Attoub S, Arafat K, Benhalilou N, Alyan S, Viallet J, Al Dhaheri Y, Eid A, Iratni R (2016) Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFkappaB and nitric oxide pathways. Sci Rep 6:21144. https://doi.org/10.1038/srep21144
Gilson P, Josa-Prado F, Beauvineau C, Naud-Martin D, Vanwonterghem L, Mahuteau-Betzer F, Moreno A, Falson P, Lafanechere L, Frachet V, Coll JL, Fernando Diaz J, Hurbin A, Busser B (2017) Identification of pyrrolopyrimidine derivative PP-13 as a novel microtubule-destabilizing agent with promising anticancer properties. Sci Rep 7(1):10209. https://doi.org/10.1038/s41598-017-09491-9
de Duve C, de Barsy T, Poole B, Trouet A, Tulkens P, Van Hoof F (1974) Commentary. Lysosomotropic agents. Biochem Pharmacol 23(18):2495–2531
Nadanaciva S, Lu S, Gebhard DF, Jessen BA, Pennie WD, Will Y (2011) A high content screening assay for identifying lysosomotropic compounds. Toxicol in Vitro 25(3):715–723. https://doi.org/10.1016/j.tiv.2010.12.010
Yoshimori T, Yamamoto A, Moriyama Y, Futai M, Tashiro Y (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H(+)-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266(26):17707–17712
Klionsky DJ, Abdelmohsen K, Abe A et al (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12 (1):1–222. https://doi.org/10.1080/15548627.2015.1100356
Logan R, Kong AC, Axcell E, Krise JP (2014) Amine-containing molecules and the induction of an expanded lysosomal volume phenotype: a structure-activity relationship study. J Pharm Sci 103(5):1572–1580. https://doi.org/10.1002/jps.23949
Logan R, Kong AC, Krise JP (2014) Time-dependent effects of hydrophobic amine-containing drugs on lysosome structure and biogenesis in cultured human fibroblasts. J Pharm Sci 103(10):3287–3296. https://doi.org/10.1002/jps.24087
Lu S, Sung T, Lin N, Abraham RT, Jessen BA (2017) Lysosomal adaptation: how cells respond to lysosomotropic compounds. PLoS One 12(3):e0173771. https://doi.org/10.1371/journal.pone.0173771
Ventola CL (2017) Cancer immunotherapy, part 3: challenges and future trends. P T 42(8):514–521
DeBord LC, Pathak RR, Villaneuva M, Liu HC, Harrington DA, Yu W, Lewis MT, Sikora AG (2018) The chick chorioallantoic membrane (CAM) as a versatile patient-derived xenograft (PDX) platform for precision medicine and preclinical research. Am J Cancer Res 8(8):1642–1660
Repnik U, Borg Distefano M, Speth MT, Ng MYW, Progida C, Hoflack B, Gruenberg J, Griffiths G (2017) L-leucyl-L-leucine methyl ester does not release cysteine cathepsins to the cytosol but inactivates them in transiently permeabilized lysosomes. J Cell Sci 130(18):3124–3140. https://doi.org/10.1242/jcs.204529
Ashoor R, Yafawi R, Jessen B, Lu S (2013) The contribution of lysosomotropism to autophagy perturbation. PLoS One 8(11):e82481. https://doi.org/10.1371/journal.pone.0082481
Hsu SPC, Kuo JS, Chiang HC, Wang HE, Wang YS, Huang CC, Huang YC, Chi MS, Mehta MP, Chi KH (2018) Temozolomide, sirolimus and chloroquine is a new therapeutic combination that synergizes to disrupt lysosomal function and cholesterol homeostasis in GBM cells. Oncotarget 9(6):6883–6896. https://doi.org/10.18632/oncotarget.23855
Chwieralski CE, Welte T, Buhling F (2006) Cathepsin-regulated apoptosis. Apoptosis 11(2):143–149. https://doi.org/10.1007/s10495-006-3486-y
Repnik U, Hafner Cesen M, Turk B (2014) Lysosomal membrane permeabilization in cell death: concepts and challenges. Mitochondrion 19 Pt A:49–57. https://doi.org/10.1016/j.mito.2014.06.006
Aits S, Jaattela M (2013) Lysosomal cell death at a glance. J Cell Sci 126(Pt9):1905–1912. https://doi.org/10.1242/jcs.091181
Uchimoto T, Nohara H, Kamehara R, Iwamura M, Watanabe N, Kobayashi Y (1999) Mechanism of apoptosis induced by a lysosomotropic agent, L-Leucyl-L-Leucine methyl ester. Apoptosis 4(5):357–362
Thiele DL, Lipsky PE (1990) Mechanism of L-leucyl-L-leucine methyl ester-mediated killing of cytotoxic lymphocytes: dependence on a lysosomal thiol protease, dipeptidyl peptidase I, that is enriched in these cells. Proc Natl Acad Sci U S A 87(1):83–87
Kornhuber J, Tripal P, Reichel M, Terfloth L, Bleich S, Wiltfang J, Gulbins E (2008) Identification of new functional inhibitors of acid sphingomyelinase using a structure-property-activity relation model. J Med Chem 51(2):219–237. https://doi.org/10.1021/jm070524a
Villamil Giraldo AM, Appelqvist H, Ederth T, Ollinger K (2014) Lysosomotropic agents: impact on lysosomal membrane permeabilization and cell death. Biochem Soc Trans 42(5):1460–1464. https://doi.org/10.1042/BST20140145
Fehrenbacher N, Bastholm L, Kirkegaard-Sorensen T, Rafn B, Bottzauw T, Nielsen C, Weber E, Shirasawa S, Kallunki T, Jaattela M (2008) Sensitization to the lysosomal cell death pathway by oncogene-induced down-regulation of lysosome-associated membrane proteins 1 and 2. Cancer Res 68(16):6623–6633. https://doi.org/10.1158/0008-5472.CAN-08-0463
ClinicalTrials.gov (2018) Study of GNS561 in Patients with liver cancer - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03316222. Accessed December 18, 2018
Acknowledgements
The authors are very grateful to Dr. Emilien Dosda, Dr. Xavier Rousset and Sylvain Roveda from Inovotion for their work on the CAM study.
Funding
This study was supported by private funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest and consent to the submission of this manuscript.
Ethical approval
According to the French legislation, no ethical approval is needed for scientific experimentations using oviparous embryos (decree n° 2013–118, February 1, 2013; art. R-214–88).
Informed consent
For this type of study, formal consent is not required. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 373 kb)
Rights and permissions
About this article
Cite this article
Brun, S., Bassissi, F., Serdjebi, C. et al. GNS561, a new lysosomotropic small molecule, for the treatment of intrahepatic cholangiocarcinoma. Invest New Drugs 37, 1135–1145 (2019). https://doi.org/10.1007/s10637-019-00741-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-019-00741-3