Summary
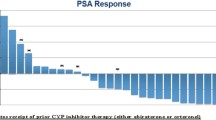
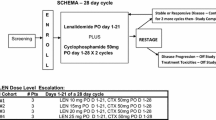
Introduction Ketoconazole is CYP-17 inhibitor with demonstrated activity in men with castration-resistant prostate cancer (CRPC). Lenalidomide is an antiangiogenic and immunomodulatory agent with broad antitumor activity. We hypothesized that the modulation of the cellular immune response to apoptosis caused by ketoconazole may be increased with the addition of lenalidomide. Methods This is an open-label, non-randomized, single-arm phase II study evaluating the efficacy and safety of the combination of ketoconazole and lenalidomide in patients with CRPC. Treatment schema included standard ketoconazole 400 mg orally three times daily plus hydrocortisone orally (20 mg in the morning and 10 mg at night) in combination with lenalidomide 25 mg orally daily for 21 days in a 28-day cycle and aspirin 75 mg daily. The primary endpoint of this study was response (either by ≥ 50% PSA decline or objective disease assessed by RECIST v1.0). Exploratory endpoints included changes in T cell, dendritic cell (DC) marker counts, and their correlation with PSA response to treatment. Results A total of 34 CRPC patients, median age 69 years, 76% ECOG 0 and 76% with metastases participated in the study. Patients received a median of 2 cycles (range 1–35); nine patients (26%) received >10 cycles of treatment. PSA responses were observed in 17 patients (50%) with 11 patients (32%) achieving a PSA decline of >90%. Among the 9 patients with measurable disease, 2 patients (22%) had PR and 2 other (22%) had SD as best response. Median time to failure (TTF) was 2.7 months (range 0.2–32.8); and 8 patients were treated for ≥ 15 months. Most common adverse events included fatigue (76%), skin reactions (62%), lymphopenia (44%) and anemia (44%). One possible treatment-related death was noted. For 16 patients with available serial correlative data, there was a significant increase in the dendritic cells subsets BDCA-1 (+146.7, −20.1 to +501.1%, p = 0.018) and BDCA-3 (39.8%, −100 to 282.6%, p = 0.001) after 8 weeks of treatment. No association between immune cell counts and PSA response at 8 weeks was observed. Conclusion The combination of ketoconazole and lenalidomide was well tolerated but did not meet the primary endpoint of response, despite durable responses were observed in a selected group of patients. Although ketoconazole has now been replaced with more active novel agents, the combination of novel CYP-17 inhibitors with agents capable of modulating the immune system warrants further prospective investigation. NCT00460031.


Similar content being viewed by others
References
National Cancer Institute (2017) Surveillance Epidemiology, and end results program. Prostate Cancer. Retrieved from http://www.seer.cancer.gov. Accessed August 2018
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30
Sharifi N, Gulley JL, Dahut WL (2005) Androgen deprivation therapy for prostate cancer. Jama 294:238–244
Cheng HH, Lin DW, Yu EY (2012) Advanced clinical states in prostate cancer. Urol Clin North Am 39:561–571
Sweeney CJ, Chen Y-H, Carducci M, Liu G, Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, Dreicer R, Vogelzang NJ, Picus J, Shevrin D, Hussain M, Garcia JA, DiPaola RS (2015) Chemohormonal therapy in metastatic hormone-sensitive prostate Cancer. N Engl J Med 373:737–746
James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, Ritchie AWS, Amos CL, Gilson C, Jones RJ, Matheson D, Millman R, Attard G, Chowdhury S, Cross WR, Gillessen S, Parker CC, Russell JM, Berthold DR, Brawley C, Adab F, Aung S, Birtle AJ, Bowen J, Brock S, Chakraborti P, Ferguson C, Gale J, Gray E, Hingorani M, Hoskin PJ, Lester JF, Malik ZI, McKinna F, McPhail N, Money-Kyrle J, O’Sullivan J, Parikh O, Protheroe A, Robinson A, Srihari NN, Thomas C, Wagstaff J, Wylie J, Zarkar A, Parmar MKB, Sydes MR (2017) Abiraterone for prostate Cancer not previously treated with hormone therapy. N Engl J Med 377:338–351
James ND, Sydes MR, Clarke NW, Mason MD, Dearnaley DP, Spears MR, Ritchie AWS, Parker CC, Russell JM, Attard G, de Bono J, Cross W, Jones RJ, Thalmann G, Amos C, Matheson D, Millman R, Alzouebi M, Beesley S, Birtle AJ, Brock S, Cathomas R, Chakraborti P, Chowdhury S, Cook A, Elliott T, Gale J, Gibbs S, Graham JD, Hetherington J, Hughes R, Laing R, McKinna F, McLaren DB, O'Sullivan JM, Parikh O, Peedell C, Protheroe A, Robinson AJ, Srihari N, Srinivasan R, Staffurth J, Sundar S, Tolan S, Tsang D, Wagstaff J, Parmar MKB (2016) Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163–1177
Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S, Protheroe A, de Porre P, Kheoh T, Park YC, Todd MB, Chi KN (2017) Abiraterone plus prednisone in metastatic, castration-sensitive prostate Cancer. N Engl J Med 377:352–360
De Coster R, Caers I, Coene MC et al (1986) Effects of high dose ketoconazole therapy on the main plasma testicular and adrenal steroids in previously untreated prostatic cancer patients. Clin Endocrinol 24:657–664
Small EJ, Baron A, Bok R (1997) Simultaneous antiandrogen withdrawal and treatment with ketoconazole and hydrocortisone in patients with advanced prostate carcinoma. Cancer 80:1755–1759
Small EJ, Baron AD, Fippin L, Apodaca D (1997) Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol 157:1204–1207
Harris KA, Weinberg V, Bok RA, Kakefuda M, Small EJ (2002) Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol 168:542–545
Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, Torti FM, Kaplan E, Vogelzang NJ (2004) Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol 22:1025–1033
Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB (2008) Lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res 14:4650–4657
LeBlanc R, Hideshima T, Catley LP et al (2004) Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood 103:1787–1790
Keizman D, Zahurak M, Sinibaldi V, Carducci M, Denmeade S, Drake C, Pili R, Antonarakis ES, Hudock S, Eisenberger M (2010) Lenalidomide in nonmetastatic biochemically relapsed prostate cancer: results of a phase I/II double-blinded, randomized study. Clin Cancer Res 16:5269–5276
Garcia JA, Elson P, Tyler A et al (2014) Sargramostim (GM-CSF) and lenalidomide in castration-resistant prostate cancer (CRPC): results from a phase I-II clinical trial. Urol Oncol 32:33.e11–37
Madan RA, Karzai FH, Ning Y-M, Adesunloye BA, Huang X, Harold N, Couvillon A, Chun G, Cordes L, Sissung T, Beedie SL, Dawson NA, Theoret MR, McLeod DG, Rosner I, Trepel JB, Lee MJ, Tomita Y, Lee S, Chen C, Steinberg SM, Arlen PM, Gulley JL, Figg WD, Dahut WL (2016) Phase II trial of docetaxel, bevacizumab, lenalidomide and prednisone in patients with metastatic castration-resistant prostate cancer. BJU Int 118:590–597
Nabhan C, Patel A, Villines D et al (2014) Lenalidomide Monotherapy in chemotherapy-naive, castration-resistant prostate Cancer patients: final results of a phase II study. Clin Genitourin Cancer 12:27–32
Wang J, McGuire TR, Britton HC et al (2015) Lenalidomide and cyclophosphamide immunoregulation in patients with metastatic, castration-resistant prostate cancer. Clin Exp Metastasis 32:111–124
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M (2008) Design and end points of clinical trials for patients with progressive prostate Cancer and castrate levels of testosterone: recommendations of the prostate Cancer clinical trials working group. J Clin Oncol 26:1148–1159
Garcia JA, Rini BI (2012) Castration-resistant prostate cancer: many treatments, many options, many challenges ahead. Cancer 118:2583–2593
Figg WD, Liu Y, Arlen P et al (2005) A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol 173:790–796
Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Vollmer R, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the prostate-specific antigen working group. J Clin Oncol 17:3461–3467
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, Antonarakis ES, Beer TM, Carducci MA, Chi KN, Corn PG, de Bono JS, Dreicer R, George DJ, Heath EI, Hussain M, Kelly WK, Liu G, Logothetis C, Nanus D, Stein MN, Rathkopf DE, Slovin SF, Ryan CJ, Sartor O, Small EJ, Smith MR, Sternberg CN, Taplin ME, Wilding G, Nelson PS, Schwartz LH, Halabi S, Kantoff PW, Armstrong AJ (2016) Trial design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials working group 3. J Clin Oncol 34:1402–1418
Costa F, Vescovini R, Bolzoni M et al (2017) Lenalidomide increases human dendritic cell maturation in multiple myeloma patients targeting monocyte differentiation and modulating mesenchymal stromal cell inhibitory properties. Oncotarget 8:53053–53067
Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB, Dalgleish AG (2009) The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother 58:1033–1045
Luptakova K, Glotzbecker B, Mills H et al (2010) Lenalidomide decreases PD-1 expression, depletes regulatory T-cells and improves cellular response to a multiple myeloma/dendritic cell fusion vaccine in vitro. Blood 116:492–492
Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN (2014) Clinical use of dendritic cells for cancer therapy. Lancet Oncol 15:e257–e267
Draube A, Klein-González N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M (2011) Dendritic cell based tumor vaccination in prostate and renal cell Cancer: a systematic review and meta-analysis. PLoS One 6:e18801
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B (2014) Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 371:424–433
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PFA, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, de Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE (2015) Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 16:152–160
Smith MR, Saad F, Chowdhury S, Oudard S, Hadaschik BA, Graff JN, Olmos D, Mainwaring PN, Lee JY, Uemura H, Lopez-Gitlitz A (2018) Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 378(15):1408–1418
Hussain M, Fizazi K, Saad F, Rathenborg P, Shore ND, Demirhan E, Modelska K, Phung D, Krivoshik A, Sternberg CN (2018). PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). J Clin Oncol 2018 36(6). https://doi.org/10.1200/JCO.2018.36.6_suppl.3
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Pedro Barata declares that he has no conflicts of interest. Matthew Cooney declares that he has no conflicts of interest. Prateek Mendiratta declares that he has no conflicts of interest. Allison Tyler declares that she has no conflicts of interest. Robert Dreicer declares that he has no conflicts of interest. Jorge A. Garcia declares that he has no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All patients provided written informed consent prior to study entry.
Rights and permissions
About this article
Cite this article
Barata, P.C., Cooney, M., Mendiratta, P. et al. Ketoconazole plus Lenalidomide in patients with Castration-Resistant Prostate Cancer (CRPC): results of an open-label phase II study. Invest New Drugs 36, 1085–1092 (2018). https://doi.org/10.1007/s10637-018-0660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0660-3