Summary
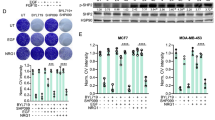
Kinases and phosphatases are important players in growth signaling and are involved in cancer development. For development of targeted cancer therapy, attention is given to kinases rather than phosphatases inhibitors. Src homology region 2 domain-containing protein tyrosine phosphatase2 (SHP2) is overexpressed in different types of cancers. We investigated the SHP2-inhibitory effects of two new 5-aminosalicylate–4-thiazolinones in human cervical (HeLa) and breast (MCF-7 & MDA-MB-231) cancer cells. In-silico molecular docking showed preferential affinity of the two compounds towards the catalytic over the allosteric site of SHP2. An enzymatic assay confirmed the docking results whereby 0.01 μM of both compounds reduced SHP2 activity to 50%. On cellular level, the two compounds significantly reduced the expression of SHP2, KRAS, p-ERK and p-STAT3 in HeLa but not in the other two cell lines. Phosphorylation of AKT and JNK was enhanced in HeLa and MCF7. Both compounds exhibited anti-proliferative/anti-migratory effects on HeLa and MCF7 but not in MDA-MB-231 cells. These results indicate that inhibition of SHP2 and its downstream pathways by the two compounds might be a promising strategy for cancer therapy in some but not all cancer types.






Similar content being viewed by others
References
Ventura J-J, Nebreda AR (2006) Protein kinases and phosphatases as therapeutic targets in cancer. Clin Transl Oncol 8:153–160. https://doi.org/10.1007/s12094-006-0005-0
Zhang J, Zhang F, Niu R (2015) Functions of Shp2 in cancer. J Cell Mol Med 19:2075–2083. https://doi.org/10.1111/jcmm.12618
Zhao S, Sedwick D, Wang Z (2014) Genetic alterations of protein tyrosine phosphatases in human cancers. Oncogene:1–10. https://doi.org/10.1038/onc.2014.326
Han T, Xiang D-M, Sun W, Liu N, Sun H-L, Wen W, Shen W-F, Wang R-Y, Chen C, Wang X, Cheng Z, Li H-Y, Wu M-C, Cong W-M, Feng G-S, Ding J, Wang H-Y (2015) PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol 63. https://doi.org/10.1016/j.jhep.2015.03.036
Grossmann KS, Rosário M, Birchmeier C, Birchmeier W (2010) The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res 106:53–89. https://doi.org/10.1016/S0065-230X(10)06002-1
Zhou XD, Agazie YM (2008) Inhibition of SHP2 leads to mesenchymal to epithelial transition in breast cancer cells. Cell Death Differ 15:988–996. https://doi.org/10.1038/cdd.2008.54
Dance M, Montagner A, Salles JP, Yart A, Raynal P (2008) The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 20:453–459. https://doi.org/10.1016/j.cellsig.2007.10.002
Butterworth S, Overduin M, Barr AJ (2014) Targeting protein tyrosine phosphatase SHP2 for therapeutic intervention. Future Med Chem 6:1423–1437. https://doi.org/10.4155/fmc.14.88
Liu Z, Zhao Y, Fang J, Cui R, Xiao Y, Xu Q, Liu Z, Zhao Y, Fang J, Cui R, Xiao Y, Xu Q (2017) SHP2 negatively regulates HLA-ABC and PD-L1 expression via STAT1 phosphorylation in prostate cancer cells. Oncotarget 8:53518–53530. https://doi.org/10.18632/oncotarget.18591
Aceto N, Sausgruber N, Brinkhaus H, Gaidatzis D, Martiny-Baron G, Mazzarol G, Confalonieri S, Quarto M, Hu G, Balwierz PJ, Pachkov M, Elledge SJ, van Nimwegen E, Stadler MB, Bentires-Alj M (2012) Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med 18:529–537. https://doi.org/10.1038/nm.2645
Wu D, Pang Y, Ke Y, Yu J, He Z, Tautz L, Mustelin T, Ding S, Huang Z, Feng GS (2009) A conserved mechanism for control of human and mouse embryonic stem cell pluripotency and differentiation by Shp2 tyrosine phosphatase. PLoS One 4. https://doi.org/10.1371/journal.pone.0004914
Chen Y-NP, LaMarche MJ, Chan HM, Fekkes P, Garcia-Fortanet J, Acker MG, Antonakos B, Chen CH-T, Chen Z, Cooke VG, Dobson JR, Deng Z, Fei F, Firestone B, Fodor M, Fridrich C, Gao H, Grunenfelder D, Hao H-X, Jacob J, Ho S, Hsiao K, Kang ZB, Karki R, Kato M, Larrow J, La Bonte LR, Lenoir F, Liu G, Liu S, Majumdar D, Meyer MJ, Palermo M, Perez L, Pu M, Price E, Quinn C, Shakya S, Shultz MD, Slisz J, Venkatesan K, Wang P, Warmuth M, Williams S, Yang G, Yuan J, Zhang J-H, Zhu P, Ramsey T, Keen NJ, Sellers WR, Stams T, Fortin PD (2016) Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 535:148–152. https://doi.org/10.1038/nature18621
Abdu Allah HH, El Shorbagi ANA (2016) 5-Aminosalyclic Acid (5-ASA): A Unique Anti-Inflammatory Salicylate. Med Chem (Los Angeles) 6. https://doi.org/10.4172/2161-0444.1000361
Abdu-Allah HHM, Abdel-Moty SG, El-Awady R, El-Shorbagi ANA (2016) Design and synthesis of novel 5-aminosalicylate (5-ASA)–4-thiazolinone hybrid derivatives with promising antiproliferative activity. Bioorg Med Chem Lett 26:1647–1650. https://doi.org/10.1016/j.bmcl.2016.02.073
Trott O, Olson A (2010) AutoDock Vina: inproving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334.AutoDock
Saleh EM, El-awady RA, Eissa NA, Abdel-Rahman WM (2012) Antagonism between curcumin and the topoisomerase II inhibitor etoposide: A study of DNA damage, cell cycle regulation and death pathways. Cancer Biol Ther 13:1058–1071. https://doi.org/10.4161/cbt.21078
Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV (2014) In vitro cell migration and invasion assays. J Vis Exp 752:e51046. https://doi.org/10.3791/51046
El-Awady RA, Saleh EM, Dahm-Daphi J (2010) Targeting DNA double-strand break repair: Is it the right way for sensitizing cells to 5-fluorouracil? Anti-Cancer Drugs 21:277–287. https://doi.org/10.1097/CAD.0b013e328334b0ae
He R, Yu Z, Zhang R, Zhang Z (2014) Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin 35:1227–1246. https://doi.org/10.1038/aps.2014.80
Prahallad A, Heynen GJJE, Germano G, Willems SM, Evers B, Vecchione L, Gambino V, Lieftink C, Beijersbergen RL, Di Nicolantonio F, Bardelli A, Bernards R (2015) PTPN11 Is a Central Node in Intrinsic and Acquired Resistance to Targeted Cancer Drugs. Cell Rep 12:1978–1985. https://doi.org/10.1016/j.celrep.2015.08.037
Schneeberger VE, Ren Y, Luetteke N, Huang Q, Chen L, Lawrence HR, Lawrence NJ, Haura EB, Koomen JM, Coppola D, Wu J (2015) Inhibition of Shp2 suppresses mutant EGFR-induced lung tumors in transgenic mouse model of lung adenocarcinoma. Oncotarget 6:6191–6202. https://doi.org/10.18632/oncotarget.3356
Eckert LB, Repasky GA, Ulkü AS, McFall A, Zhou H, Sartor CI, Der CJ (2004) Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res 64:4585–4592. https://doi.org/10.1158/0008-5472.CAN-04-0396
Perera D, Venkitaraman AR (2016) Oncogenic KRAS triggers MAPK-dependent errors in mitosis and MYC-dependent sensitivity to anti-mitotic agents. Sci Rep 6. https://doi.org/10.1038/srep29741
Mendoza MC, Er EE, Blenis J (2011) The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem Sci 36:320–328. https://doi.org/10.1016/j.tibs.2011.03.006
Li X, Lu Y, Liang K, Liu B, Fan Z (2005) Differential responses to doxorubicin-induced phosphorylation and activation of Akt in human breast cancer cells. Breast Cancer Res 7:R589–R597. https://doi.org/10.1186/bcr1259
Li X, Ma H, Li L, Chen Y, Sun X, Dong Z, Liu JY, Zhu W, Zhang JT (2018) Novel synthetic bisindolylmaleimide alkaloids inhibit STAT3 activation by binding to the SH2 domain and suppress breast xenograft tumor growth. Oncogene. https://doi.org/10.1038/s41388-017-0076-0
Magi S, Tashiro E, Imoto M (2012) A chemical genomic study identifying diversity in cell migration signaling in cancer cells. Sci Rep 2. https://doi.org/10.1038/srep00823
Funding
The work was supported by AlJalila Foundation for Medical Education and Research, United Arab Emirates [grant number. AJF201612]
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Author CV declares that he has no conflict of interest. Author ES declares that he has no conflict of interest. Author WR declares that he has no conflict of interest. Author VM declares that he has no conflict of interest. Author AA declares that he has no conflict of interest. Author HT declares that he has no conflict of interest. Author HA declares that he has no conflict of interest. Author AE declares that he has no conflict of interest. Author RE declares that he has no conflict of interest
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Vazhappilly, C., Saleh, E., Ramadan, W. et al. Inhibition of SHP2 by new compounds induces differential effects on RAS/RAF/ERK and PI3K/AKT pathways in different cancer cell types. Invest New Drugs 37, 252–261 (2019). https://doi.org/10.1007/s10637-018-0626-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-018-0626-5