Summary
Background Progress in developing effective salvage therapies for UC is warranted. Alisertib is an orally available, selective inhibitor of the aurora kinase A. Methods A single-group, phase 2 trial was conducted with alisertib 50 mg orally BID for 7 days, with 14d rest until disease progression (PD) (NCT02109328). The primary endpoint (EP) was RECIST 1.1 objective response-rate (ORR, H0 ≤ 5 %, H1 ≥ 20 %, α = 10 % and β = 20 %). Eligibility included failure of at least one platinum-based regimen. Results From 10/2014 to 04/2015, 22 patients were enrolled (20 evaluable for response), 8 (36.4 %) in second-line and 14 (63.6 %) beyond the second-line. Eight (36.4 %) had an ECOG-performance status 1–2. Two partial responses (PR, ORR: 9.1 %), 7 stable disease (SD) and 11 PD were obtained. Median follow-up was 8.3 months (IQR: 7–10.3), 6-month progression-free survival (PFS) was 13.6 % (95%CI: 4.8–39.0). Two SD are still receiving treatment after 11.5 and 6.3 months. Median overall survival (OS) was not reached (6-month OS: 59.1 %, 95%CI: 41.7–83.7). Hb < 10 g/dl was significantly associated with shorter PFS and OS multivariably (p = 0.031 and p = 0.033). Tissue of the case with 11.5 month SD harbored a missense mutation of mTOR (E1813D), the nonsense mutation Q527STOP of TSC1, HER3 and TAF1L missense mutations. Grade 3–4 adverse events (AE) were: 40.9 % mucositis, 36.4 % fatigue, 18.2 % neutropenia (13.6 % febrile neutropenia). There were 2 treatment-related deaths. Conclusions The study did not meet the primary EP, yet sustained disease control was obtained in about 14 % of patients. The incidence of AE and the issue of patient selection are two major concerns.



Similar content being viewed by others
References
Powles T, Eder JP, Fine GD, et al. (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562
Plimack ER, Bellmunt J, Gupta S, et al. (2015) Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from KEYNOTE-012. J Clin Oncol 33 (suppl; abstr 4502).
Petrylak DP, Powles T, Bellmunt J, et al. (2015) A phase Is study of MPDL3280A (anti-PDL1): updated response and survival data in urothelial bladder cancer (UBC). J Clin Oncol 33 (suppl; abstr 4501).
Rosenberg J, Petrylak D, Abidoye O, et al. (2015) (21LBA) Atezolizumab in patients (pts) with locally-advanced or metastatic urothelial carcinoma (mUC): results from a pivotal multicenter phase II study (IMvigor 210). Eur J Cancer 51(Supplement 3):S720
Sonpavde G, Jones BS, Bellmunt J, et al. (2015) Future directions and targeted therapies in bladder cancer. Hematol Oncol Clin North Am 29:361–376
Raggi D, Miceli R, Sonpavde G, et al. (2015) Second-line single-agent versus doublet chemotherapy as salvage therapy for metastatic urothelial cancer: a systematic review and meta-analysis. Ann Oncol. doi:10.1093/annonc/mdv509
Sonpavde G, Pond GR, Choueiri TK, et al. (2015) Single agent taxane versus taxane containing combination chemotherapy as salvage therapy for advanced urothelial carcinoma. Eur Urol. doi:10.1016/j.eururo.2015.07.042
Sen S, Zhou H, Zhang RD, et al. (2002) Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst 94:1320–1329
Zhou N, Singh K, Mir MC, et al. (2013) The investigational Aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell cycle progression in bladder cancer cells in vitro and in vivo. Clin Cancer Res 19:1717–1728
Cervantes A, Elez E, Desamparados R, et al. (2012) Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective Aurora A kinase inhibitor, in aptients with advanced solid tumors. Clin Cancer Res 18:4764–4774
Eisenhauer EA, Therasse P, Bogaerts J, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Young H, Baum R, Cremerius U, et al. (1999) Measurement of clinical and subclinical tumour response using (18F)-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for the research and treatment of cancer (EORTC) PET study group. Eur J Cancer 35:1773–1782
Melichar B, Adenis A, Lockhart AC, et al. (2015) Safety and activity of alisertib, an investigational Aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous cell carcinoma, and gastro-oesophageal adenocarcinoma: five-arm phase 2 study. Lancet Oncol 16:395–405
Barr PM, Li H, Spier C, et al. (2015) Phase II intergroup trial of alisertib in relapsed and refractory peripheral T-cell lymphoma and transformed mycosis fungoides: SWOG 1108. J Clin Oncol 33:2399–2404
Friedberg JW, Mahadevan D, Cebula E, et al. (2013) Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol 32:44–50
Matulonis UA, Sharma S, Ghamande S, et al. (2012) Phase II study of MLN8237 (alisertib), an investigational Aurora A kinase inhibitor, in patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol 127:63–69
Stadler WM, Vaughn DJ, Sonpavde G, et al. (2014) An open-label, single-arm, phase 2 trial of the polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer 120:976–982
Bellmunt J, Choueiry TK, Fougeray R, et al. (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28:1850–1855
Sonpavde G, Pond GR, Fougeray R, et al. (2013) Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol 63:717–723
Sonpavde G, Pond GR, Rosenberg JE, et al. (2015) Improved 5-Factor Prognostic Classification of Patients Receiving Salvage Systemic Therapy for Advanced Urothelial Carcinoma. J Urol. doi:10.1016/j.juro.2015.07.111
Iyer G, Hanrahan AJ, Milowsky MI, et al. (2012) Genome sequencing identifies a basis for everolimus sensitivity. Science 338:221
Wagle N, Grabiner BC, Van Allen EM, et al. (2014) Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov 4:546–553
Tentler JJ, Iokina AA, Tan AC, et al. (2015) p53 family members regulate phenotypic response to Aurora kinase A inhibition in triple-negative breast cancer. Mol Cancer Ther 14:1117–1129
Marxer M, Ma HT, Poon RYC (2014) p53 deficiency enhances mitotic arrest and slippage induced by pharmacological inhibition of Aurora kinases. Oncogene 33:3550–3560
Tabernero J, Bahleda R, Dientsmann R, et al. (2015) Phase I dose escalation study of JNJ-42756493, an oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced solid tumors. J Clin Oncol 33:3401–3408
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The study was partially supported by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Next generation sequencing analyses were supported in part by funds obtained through an Italian law that allows taxpayers to allocate 0.5 % share of their income tax contribution to a research Institution of their choice.
Electronic supplementary material
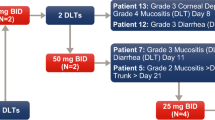
Supplementary Figure 1
Design of the study. Abbreviations: BID: bis-in-die (twice daily); PFS: progression-free survival. (JPEG 509 kb)
Supplementary Table 1
(DOCX 87 kb)
Supplementary Table 2
(DOCX 87 kb)
Rights and permissions
About this article
Cite this article
Necchi, A., Lo Vullo, S., Mariani, L. et al. An open-label, single-arm, phase 2 study of the Aurora kinase A inhibitor alisertib in patients with advanced urothelial cancer. Invest New Drugs 34, 236–242 (2016). https://doi.org/10.1007/s10637-016-0328-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0328-9