Summary
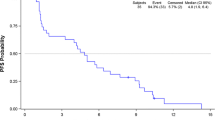
Background This phase I trial assessed safety, pharmacokinetics (PK), dose limiting toxicity (DLT), maximum tolerated dose and recommended dose (RD) of the combination of sorafenib plus ifosfamide in patients with advanced sarcoma. Methods Twelve sarcoma patients (9 soft-tissue, 3 bone sarcoma) were treated with sorafenib plus ifosfamide (starting doses 200 mg bid and 6 g/m2 respectively). A 3 + 3 dose escalation design with cohorts of 3–6 patients was used. A study to assess the in vitro efficacy of the combination was also conducted. Results Three DLTs were observed: fatigue grade 4 with sorafenib 400 mg bid plus ifosfamide 6 g/m2 and encephalopathy and emesis grade 3 with sorafenib 400 mg bid plus ifosfamide 7.5 g/m2. Other toxicities included diarrhea, hand-foot syndrome, mucositis, neutropenia, skin rash and thrombocytopenia. There were no relevant effects on PK of sorafenib but an increase in ifosfamide active metabolite 4-hydroxy-ifosfamide was observed. Eight patients achieved stable disease lasting more than 12 weeks. An additive effect was observed in vitro. Conclusions RD was sorafenib 400 mg bid plus ifosfamide 6 g/m2, allowing administration of active doses of both agents. Limited preliminary antitumor activity was also observed. A phase II study is currently ongoing.


Similar content being viewed by others
References
O’Bryan RM, Luce JK, Talley RW, Gottlieb JA, Baker LH, Bonadonna G (1973) Phase II evaluation of adriamycin in human neoplasia. Cancer 32(1):1–8
Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, Parkinson DR (1993) Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11(7):1269–1275
Santoro A, Tursz T, Mouridsen H, Verweij J, Steward W, Somers R, Buesa J, Casali P, Spooner D, Rankin E (1995) Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 13(7):1537–1545
Judson I, Radford JA, Harris M, Blay JY, van Hoesel Q, le Cesne A, van Oosterom AT, Clemons MJ, Kamby C, Hermans C, Whittaker J, Donato di Paola E, Verweij J, Nielsen S (2001) Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer 37(7):870–877
Buesa JM, Lopez-Pousa A, Martin J, Antón A, García del Muro J, Bellmunt J, Arranz F, Valentí V, Escudero P, Menéndez D, Casado A, Poveda A (1998) Phase II trial of first-line high-dose ifosfamide in advanced soft tissue sarcomas of the adult: a study of the Spanish Group for Research on Sarcomas (GEIS). Ann Oncol 9(8):871–876
van Oosterom AT, Mouridsen HT, Nielsen OS, Dombernowsky P, Krzemieniecki K, Judson I, Svancarova L, Spooner D, Hermans C, Van Glabbeke M, Verweij J, EORTC Soft Tissue and Bone Sarcoma Group (2002) Results of randomized studies of the EORTC Soft Tissue and Bone Sarcoma Group (STBSG) with two different ifosfamide regimens in first- and second-line chemotherapy in advanced soft tissue sarcoma patients. Eur J Cancer 38(18):2397–2406
Buesa JM, Mouridsen HT, van Oosterom AT, Verweij J, Wagener T, Steward W, Poveda A, Vestlev PM, Thomas D, Sylvester R (1991) High-dose DTIC in advanced soft-tissue sarcomas in the adult. A phase II study of the E.O.R.T.C. Soft Tissue and Bone Sarcoma Group. Ann Oncol 2(4):307–309
Garcia Del Muro X, Lopez-Pousa A, Martin J, Buesa JM, Martinez-Trufero J, Casado A, Poveda A, Cruz J, Bover I, Maurel J (2005) A phase II trial of temozolomide as a 6-week, continuous, oral schedule in patients with advanced soft tissue sarcoma: a study by the Spanish Group for Research on Sarcomas. Cancer 104(8):1706–1712
Graeven U, Andre N, Achilles E, Zornig C, Schmiegel W (1999) Serum levels of vascular endothelial growth factor and basic fibroblast growth factor in patients with soft-tissue sarcoma. J Cancer Res Clin Oncol 125(10):577–581
Kuhnen C, Lehnhardt M, Tolnay E, Muehlberger T, Vogt PM, Müller KM (2000) Patterns of expression and secretion of vascular endothelial growth factor in malignant soft-tissue tumours. J Cancer Res Clin Oncol 126(4):219–225
Borgstrom P, Hillan KJ, Sriramarao P, Ferrara N (1996) Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res 56(17):4032–4039
Fong TA, Shawver LK, Sun L, Tang C, App H, Powell TJ, Kim YH, Schreck R, Wang X, Risau W, Ullrich A, Hirth KP, McMahon G (1999) SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res 59(1):99–106
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
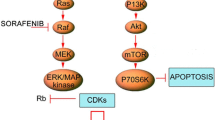
Ambrosini G, Cheema HS, Seelman S, Teed A, Sambol EB, Singer S, Schwartz GK (2008) Sorafenib inhibits growth and mitogen-activated protein kinase signaling in malignant peripheral nerve sheath cells. Mol Cancer Ther 7(4):890–896
Maruwge W, D’Arcy P, Folin A, Brnjic S, Wejde J, Davis A, Erlandsson F, Bergh J, Brodin B (2008) Sorafenib inhibits tumor growth and vascularization of rhabdomyosarcoma cells by blocking IGF-1R-mediated signaling. Onco Targets Ther 1:67–78
Pignochino Y, Grignani G, Cavalloni G, Motta M, Tapparo M, Bruno S, Bottos A, Gammaitoni L, Migliardi G, Camussi G, Alberghini M, Torchio B, Ferrari S, Bussolino F, Fagioli F, Picci P, Aglietta M (2009) Sorafenib blocks tumour growth, angiogenesis and metastatic potential in preclinical models of osteosarcoma through a mechanism potentially involving the inhibition of ERK1/2, MCL-1 and ezrin pathways. Mol Cancer 8:118
Peng CL, Guo W, Ji T, Ren T, Yang Y, Li DS, Qu HY, Li X, Tang S, Yan TQ, Tang XD (2009) Sorafenib induces growth inhibition and apoptosis in human synovial sarcoma cells via inhibiting the RAF/MEK/ERK signaling pathway. Cancer Biol Ther 8(18):1729–1736
Lu X, Tang X, Guo W, Ren T, Zhao H (2010) Sorafenib induces growth inhibition and apoptosis of human chondrosarcoma cells by blocking the RAF/ERK/MEK pathway. J Surg Oncol 102(7):821–826
Maki RG, D’Adamo DR, Keohan ML, Saulle M, Schuetze SM, Undevia SD, Livingston MB, Cooney MM, Hensley ML, Mita MM, Takimoto CH, Kraft AS, Elias AD, Brockstein B, Blachère NE, Edgar MA, Schwartz LH, Qin LX, Antonescu CR, Schwartz GK (2009) Phase II study of sorafenib in patients with metastatic or recurrent sarcomas. J Clin Oncol 27(19):3133–3140
Pacey S, Ratain MJ, Flaherty KT, Kaye SB, Cupit L, Rowinsky EK, Xia C, O’Dwyer PJ, Judson IR (2011) Efficacy and safety of sorafenib in a subset of patients with advanced soft tissue sarcoma from a Phase II randomized discontinuation trial. Invest New Drugs 29(3):481–488
Grignani G, Palmerini E, Dileo P, Asaftei SD, D’Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F, Casali PG, Ferrari S, Aglietta M (2012) A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 23(2):508–516
Vincent P, Zhang X, Chen C, Lantz L, Rembiesa C, Polony B, Carter CA, Bayer Research Center, West Haven, CT et al. (2002) Chemotherapy with the raf kinase inhibitor BAY 43–9006 in combination with irinotecan, vinorelbine, or gemcitabine is well tolerated and efficacious in preclinical xenograft models Proc Am Soc Clin Oncol 21 (abstr 1900).
D’Adamo DR, Anderson SE, Albritton K, Yamada J, Riedel E, Scheu K, Schwartz GK, Chen H, Maki RG (2005) Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol 23(28):7135–7142
George S, Merriam P, Maki RG, Van den Abbeele AD, Yap JT, Akhurst T, Harmon DC, Bhuchar G, O’Mara MM, D’Adamo DR, Morgan J, Schwartz GK, Wagner AJ, Butrynski JE, Demetri GD, Keohan ML (2009) Multicenter phase II trial of sunitinib in the treatment of nongastrointestinal stromal tumor sarcomas. J Clin Oncol 27(19):3154–3160
Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, Collin F, Pandite L, Marreaud S, De Brauwer A, van Glabbeke M, Verweij J, Blay JY (2009) Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043). J Clin Oncol 27(19):3126–3132
van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP, Beppu Y, Le Cesne A, Gelderblom H, Judson IR, Araki N, Ouali M, Marreaud S, Hodge R, Dewji MR, Coens C, Demetri GD, Fletcher CD, Dei Tos AP, Hohenberger P, EORTC Soft Tissue and Bone Sarcoma Group, PALETTE study group (2012) Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379(9829):1879–1886
Hamberg P, Steeghs N, Loos WJ, van de Biessen D, den Hollander M, Tascilar M, Verweij J, Gelderblom H, Sleijfer S (2010) Decreased exposure to sunitinib due to concomitant administration of ifosfamide: results of a phase I and pharmacokinetic study on the combination of sunitinib and ifosfamide in patients with advanced solid malignancies. Br J Cancer 102(12):1699–1706
van Glabbeke M, Verweij J, Judson I, Nielsen OS, EORTC Soft Tissue and Bone Sarcoma Group (2002) Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 38(4):543–549
Acknowledgments
This work was supported in part by Bayer España.
Disclosures
E. Brendel is an employee of Bayer Pharma AG in the department of Clinical Sciences/Clinical Pharmacology. The rest of the authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martín-Liberal, J., López-Pousa, A., Broto, J.M. et al. Phase I trial of sorafenib in combination with ifosfamide in patients with advanced sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs 32, 287–294 (2014). https://doi.org/10.1007/s10637-013-9989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-013-9989-9