Summary
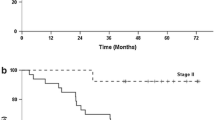
Purpose The feasibility of a 3-week combination of S-1 and cisplatin as an adjuvant chemotherapy for patients with curatively resected gastric cancer was investigated. Experimental design Korean patients with stage II-IV (M0) gastric adenocarcinoma who underwent a gastrectomy with D2 lymph node resection were enrolled. The S-1 was administered orally at 80 mg/m2 divided into two daily doses for 14 days, while the cisplatin was administered at 60 mg/m2 intravenously over 2 h every 21 days. The patients received a maximum of six cycles. Results From January 2006 to July 2010, 74 patients were included in this study. The median patient age was 56 years (range, 22–71), and 51.4% (38/74) of the patients had a performance status of 0. The median number of chemotherapy cycles administered was 6 (range, 1–6). The median relative dose intensity was 86.4% for S-1 and 80.0% for cisplatin. With a median follow-up duration of 13.9 months, the median relapse-free survival (RFS) and overall survival (OS) have not yet been reached. Fifteen relapses (20.3%) were documented. Plus, the estimated RFS rate was 60.5% at 3 years. The treatments were generally well tolerated. The most frequently observed grade 3–4 hematological toxicity was neutropenia (35.1%), and only 1 cycle of neutropenic fever occurred. The most frequently observed grade 3–4 non-hematological toxicities were nausea (4.1%) and asthenia (4.1%), and all the other grade 3–4 non-hematological toxicities were observed in less than 3% of the patients. Conclusions Postoperative adjuvant S-1 plus cisplatin for 18 weeks was found to be feasible for patients with stage II-IV (M0) gastric adenocarcinoma following complete surgical resection.


Similar content being viewed by others
References
Oba K (2009) Efficacy of adjuvant chemotherapy using tegafur-based regimen for curatively resected gastric cancer: update of a meta-analysis. Int J Clin Oncol 14:85–89
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, Martenson JA (2001) Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, Smith DB, Langley RE, Verma M, Weeden S, Chua YJ, MAGIC Trial Participants (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11–20
Kubota T (2008) The role of S-1 in the treatment of gastric cancer. Br J Canc 98:1301–1304
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357:1810–1820
Koizumi W, Narahara H, Hara TT, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kodera Y, Ishiyama A, Yoshikawa T, Kinoshita T, Ito S, Yokoyama H, Mochizuki Y, Ito H, Tsuburaya A, Sakamoto J, Nakao A (2010) A feasibility study of postoperative chemotherapy with S-1 and cisplatin (CDDP) for gastric carcinoma (CCOG0703). Gastric Canc 13:197–203
Lee JL, Kang HJ, Kang YK, Ryu MH, Chang HM, Kim TW, Sohn HJ, Kim H, Lee JS (2008) Phase I/II study of 3-week combination of S-1 and cisplatin chemotherapy for metastatic or recurrent gastric cancer. Canc Chemother Pharmacol 61:837–845
Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, Kinoshita T, Yasui H, Terashima M, Goto M, Tanigawa N, Shirao K, Sano T, Sasako M (2010) Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Canc Chemother Pharmacol
Greene FL (2003) TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol 21:23–29
Saif MW, Syrigos KN, Katirtzoglou NA (2009) S-1: a promising new oral fluoropyrimidine derivative. Expet Opin Investig Drugs 18:335–348
Hejna M, Wohrer S, Schmidinger M, Raderer M (2006) Postoperative chemotherapy for gastric cancer. Oncologist 11:136–145
Shirasaka T, Shimamoto Y, Ohshimo H, Saito H, Fukushima M (1993) Metabolic basis of the synergistic antitumor activities of 5-fluorouracil and cisplatin in rodent tumor models in vivo. Canc Chemother Pharmacol 32:167–172
De Vita F, Giuliani F, Galizia G, Belli C, Aurilio G, Santabarbara G, Ciardiello F, Catalano G, Orditura M (2007) Neo-adjuvant and adjuvant chemotherapy of gastric cancer. Ann Oncol 18 Suppl 6:vi120–vi123
De Vita F, Vecchione L, Galizia G, Di Martino N, Fabozzi T, Catalano G, Ciardiello F, Orditura M (2009) Perspectives in adjuvant therapy of gastric cancer. Oncology 77 Suppl 1:38–42
Lee SS, Jeung HC, Chung HC, Noh SH, Hyung WJ, Ahn JY, Rha SY (2010) A pilot study of S-1 plus cisplatin versus 5-fluorouracil plus cisplatin for postoperative chemotherapy in histological stage IIIB-IV (M0) gastric cancer. Investig New Drugs
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, Shirao K, Matsumura Y, Gotoh M (2003) Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Canc 89:2207–2212
Acknowledgments
All the authors collected the data, interpreted the data, and contributed to the manuscript in collaboration. The authors also reported no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jong Gwang Kim and Wansik Yu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kang, B.W., Kim, J.G., Chae, Y.S. et al. Pilot study of adjuvant chemotherapy with 3-week combination of S-1 and cisplatin for patients with stage II-IV (M0) gastric cancer. Invest New Drugs 30, 1671–1675 (2012). https://doi.org/10.1007/s10637-011-9729-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9729-y