Summary
Cancer stem cells (CSC) are chemoresistant and implicated in tumor recurrence, metastasis and high patient mortality; thus substances impairing CSC activity, could be invaluable as novel cancer therapeutics. We previously showed that CAPE (caffeic acid phenethyl ester), a component of propolis, a honeybee product, inhibits growth of MDA-MB-231 (MDA-231) cells, mdr gene expression, NF-κB, EGFR, and VEGF. We hypothesized that CAPE also acts by interfering with CSC-mediated effects. We isolated breast CSC (bCSC) from MDA-231 cells, a model of human triple-negative breast cancer, and mouse xenografts. bCSC grow as mammospheres (MMS) and when dissociated into single cells, form MMS again, a sign of self-renewal. bCSC exhibited the characteristic CD44+/CD24-/low phenotype and generated progenitors in the presence of serum, a CSC trait responsible for regenerating tumor mass. CAPE caused dose-dependent bCSC self-renewal inhibition and progenitor formation. Clonal growth on soft agar was inhibited dose-dependently, but apoptosis was not induced as determined by Annexin-V/PI assay. Instead, bCSC were noted to significantly progress from a quiescent cell cycle state in G0/G1 (82%), S phase (12%) to a cycling state with an increase in S phase (41%) and subsequent decrease in G0/G1 (54%). Treatment of bCSC with CAPE (4.5-days) decreased CD44 levels by 95%, while another cell population containing 10-100-fold lower CD44 content concurrently increased. Results suggest that CAPE causes pronounced changes in bCSC characteristics manifested by inhibition of self renewal, progenitor formation, clonal growth in soft agar, and concurrent significant decrease in CD44 content, all signs of decreased malignancy potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A subset of cells in tumors called cancer stem cells (CSCs) or cancer initiating cells are found in many solid tumor types, including breast cancer and can give rise to the differentiated cells that make up the tumor bulk [1–4]. Suspension sphere cultures have been used to enrich for and characterize stem/progenitor cells from primary breast and glioblastomic tissues [5–7]. This process is thought to simulate the events of tissue regeneration and maintenance from cells that survive suspension conditions. In human breast cancers, these tumorigenic breast cancer stem cells (bCSC) are enriched in cells with a CD44+/CD24-/low/ESA+ phenotype [1]. CD44+/CD24-/low/ESA+ cells exhibit properties of self-renewal in vitro, form tumors from very few cells, divide slowly, and are selectively resistant to chemotherapy, all of which are hallmarks of cancer stem cells [8].
CD44 acts as a receptor for hyaluronan. Hyaluronan (HA) is a high molecular weight glycosaminoglycan, extracellular matrix component essential for proper cell growth, organ structural stability, and tissue organization [9]. HA acts through CD44, its principal receptor, and RHAMM (receptor for HA mediated motility) to regulate cell proliferation and movement [10]. Many of the downstream pathways following CD44 activation become deregulated in cancer, leading to tumor growth, progression and metastasis [11–13]. Changes in both CD44 and HA expression have been widely observed in tumors from cancer patients and occur in animal models of tumor growth [14]. During carcinogenesis, expression of the standard form of CD44 is upregulated in certain cancers. Interference with the CD44-HA interaction by either targeting drugs to CD44, to the HA matrix or interfering with HA matrix-CD44 interactions are viable strategies for cancer treatments [15–17].
Caffeic acid phenethyl ester (CAPE) is one of the main medicinal components of propolis, a honeybee-derived natural product. CAPE possesses important biological activities, including antibacterial, antiviral, antioxidant, anti-inflammatory, and anti-cancer [18–21]. CAPE inhibits nuclear factor kappa B (NF-κB) and induces apoptosis [22–24]. CAPE’s anticancer effects have been demonstrated in a number of cancer models [25–34], including our data on breast cancer [35–39]. We have previously shown that CAPE inhibits mdr gene expression, NF-κB, EGFR, and VEGF. CAPE inhibits growth of MCF-7 and MDA-231 cells in vitro and in vivo; in particular, CAPE optimally inhibited MDA-231 xenografts at even lower doses than those required for inhibition of MCF-7-induced tumors ([36], Wu J, Omene C et al., manuscript submitted).
In this study, we investigated the effect of CAPE on breast cancer stem cells derived from the triple negative breast cancer (TNBC) model, MDA-231 cells, an aggressive subtype of breast cancer and analyzed the effects on bCSC’s self renewal, progenitor formation, and the CD44 cell marker phenotype, cell cycle, and apoptosis.
Materials and methods
Mammosphere culture and bCSC isolation
MDA-MB-231 (MDA-231) cells were cultured in Leibovitz L-15 medium (LB-15) (Cellgro, Manassas, VA) supplemented with 5% FBS (Cellgro, Manassas, VA). Floating cells were collected and cultured in six-well Ultra Low Attachment (ULA) plates (Corning, NY) using 3 × 104 cells per well in 3 ml serum-free mammosphere medium [LB15-mm: LB-15 containing 1:50 B27 (Invitrogen Ltd.) and 20 ng/ml epidermal growth factor (EGF; R&D Systems, Inc.)]. Cells were fed every 3 days and passaged using 0.05% trypsin-0.53 mmol/L EDTA-Na4. The MDA-231 cell line and cells derived from MDA-231-induced nude mouse xenografts were cultured in LB-15 medium+5% FBS. bCSC were grown as MMS in LB15-mm medium in the absence of serum, as described above. MMS were used for experiments after 4 weeks of growth in culture.
CSC surface marker characterization
Mammospheres derived from MDA-231 cells were dissociated with trypsin-EDTA to form single-cell suspensions. Cells were washed, blocked by 1X PBS in the presence of 2% BSA and resuspended in wash buffer (106cells/100 μl). Fluorochrome-conjugated monoclonal antibodies (BD Bioscience) against human CD44 (APC) and CD24 (PE) were added to the cell suspensions according to the manufacturer instructions and incubated at 4°C in the dark for 30–40 min. The labeled cells were washed and fixed in PBS containing 1% paraformaldehyde before analysis by flow cytometry on FACScalibur (Becton Dickinson).
Cell viability and apoptosis assays
Mammospheres were grown in serum-free MMS medium (LB15-mm). Following passaging, MMS were allowed to settle via gravity in a 15 ml centrifuge tube for 20–30 min and the supernatant was gently discarded. After complete removal of a supernatant, MMS were dissociated into single cells by incubation with trypsin-EDTA at 37°C for 3 min and light vortexing. After diluting trypsin with LB-15 medium, cells were pelleted (1,500 rpm/5 min) and resuspended in LB15-mm. Single cell suspensions were seeded into 6-well ULA plates (3 × 104/3 ml LB15-mm/well), and CAPE (Sigma-Aldrich) in DMSO (3-μl stock solution) or 3-μl DMSO were added/well; the latter as a vehicle control. Equal volume of DMSO was added to controls for a final DMSO concentration of 0.1%. After 4.5 days, MMS were collected, dissociated by trypsin-EDTA to single cells, and cells were counted using trypan blue exclusion assay.
In other bCSC viability experiments, CAPE-pretreated or untreated MMS were seeded in regular, cell adherent 96-well plates (Corning, NY), at densities of 800, 400, 200, and 100 cells/well in 200 μL of LB-15 medium containing 5% FBS. After 6 days, 10 μl of 5 mg/ml MTT, a tetrazolium dye colorimetric test were added into each well and incubated for 4 h. DMSO was used to dissolve the purple formazan crystals inside the cells. At the end of incubation, cell viability was assessed as indicated by the conversion of tetrazolium salts to a colored product formazan, the concentration of which was measured spectrophotometrically to calculate the IC50 value.
For apoptosis experiments, 3 × 104/3 ml LB15-mm/well cells were seeded and incubated with CAPE for 4.5 days. Cells were harvested, washed twice with PBS, and centrifuged. 1 × 104 cells per sample were treated with Annexin V-FITC and propidium iodide (PI) using the apoptosis Detection Kit (BD Bioscience Pharmingen, San Jose, CA) according to the manufacturer’s protocol. Annexin V-FITC and PI binding were analyzed by flow cytometry without gating restrictions using 5,000 cells. Data were collected using logarithmic amplification of both the FL1 (FITC) and FL2 (PI) channels. Quadrant analysis of co-ordinate dot plots was performed with CellQuest software. Unstained cells were used to adjust the photomultiplier voltage and for compensation setting adjustment in order to eliminate spectral overlap between the FL1 and FL2 signals.
Cell cycle analyses
Single-cell suspension cells derived from MMS were seeded as above and treated with either 0 μM or 40 μM CAPE for 48 h. Samples were then collected, prepared as single-cell suspensions, and fixed in cold 70% ethanol for 24 h at −20°C. Fixed cells were washed with 1XPBS and stained with propidium iodide solution containing RNAse A at 10 μg/ml before analysis by flow cytometry.
Clonal growth on soft agar
3 × 104 single-cell suspensions isolated from MDA-231’s MMS were seeded in 6-well ULA plates in 3 ml LB-15 mm medium and treated with different concentrations of CAPE for 2 weeks. After treatment, MMS were collected and dissociated to single cell suspensions. 1 × 104 cells were then seeded onto soft agar for 1 week or 4 weeks in the presence of 5% FBS and clones were counted under an inverted microscope. Clones with ≥50 cells together are counted as one clone. Clones did not grow in the absence of FBS.
Results
bCSC isolation and characterization (Fig. 1)
MDA-231-derived bCSC were successfully isolated from both MDA-231 cell–induced xenografts in nude female mice and from the cultured MDA-231 cells and were propagated in culture in the form of mammospheres (MMS) (Fig. 1a). When dissociated into single cells, mammospheres, with time, formed MMS again. This process was repeated for many generations and is considered a sign of self-renewal. These cells exhibited the characteristic CD44+/CD24-/low phenotype where up to 75% of the cell population stained for this bCSC phenotype (Fig. 1b).
bCSC isolation and characterization: bCSC were isolated from both MDA-231 cells and MDA-231 cell-induced nude mouse xenografts and propagated in culture in the form of mammospheres (MMS) (a). 75% of these cells exhibited the characteristic CD44+/CD24-/low staining (Region 8, lower-right quadrant) of the bCSC phenotype (b)
Effect of CAPE on progenitors and differentiated cells derived from MMS (Fig. 2)
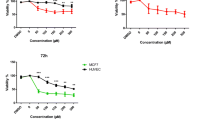
Single-cell suspensions derived from MMS were treated in the absence of serum with CAPE at different concentrations from 0 to 40 μM and, after 4.5 days, viable cells were counted. CAPE inhibited MMS formation in a dose dependent manner with a 97% inhibition at 40 μM CAPE (Fig. 2a). Single-cell suspensions derived from MMS pre-treated with CAPE for 4.5 days or untreated were seeded at densities of 800, 400, 200, and 100 cells/well of 96-well plate in LB-15 containing 5% FBS without CAPE. After 6 days, cell growth was analyzed by MTT assay. In the absence of CAPE (0 μM), the bCSCs had the ability to form progenitors and differentiated cells in the presence of serum (the term progenitors will be used henceforth) and this ability was cell concentration-dependent with viability increasing at higher bCSC cell densities. However, progenitor formation in the presence of serum was inhibited up to 53% at the highest bCSC density of 800 cells/well at a CAPE concentration of 20 μM (Fig. 2b).
CAPE inhibits MMS and progenitor formation: CAPE treatment inhibits MMS growth (without serum, a) and progenitor formation (with serum, b) in a dose-dependent manner. This inhibition was statistically significant at 400 cells/well (*p = 0.0002) and 800 cells/well (**p = 0.004), b The experimental differences were determined by two-tailed Student’s t-test; n = 3 and p ≤ 0.05 was taken as a significant difference
CAPE effect on MMS growth in soft agar (Fig. 3)
Mammospheres were pre-treated with different concentrations of CAPE for 2 weeks then dissociated into single cells and equal numbers of viable cells were seeded onto soft agar for 1 or 4 weeks growth in the presence of serum (Clones did not grow in agar in the absence of 5% FBS). CAPE pre-treatment resulted in the inhibition of mammosphere growth in soft agar as seen by decreases in the MMS sizes as shown in photographs in Fig. 3a. CAPE exhibited both a dose-dependent and a time-dependent inhibitory effect with a decline from ~100% clones at 0 μM CAPE to~18% clones at 40 μM CAPE after 1 week of growth, and to <5% clones at 40 μM CAPE after 4 weeks of growth on soft agar (Fig. 3b).
CAPE inhibits mammosphere growth on soft agar: Single cells derived from CAPE pre-treated MMS (2 weeks) were seeded onto soft agar for 1 or 4 weeks in the presence of serum. CAPE pre-treatment resulted in a dose-dependent inhibition of MMS growth on soft agar (a) as clonal growth declined from ~100% clones at 0 μM CAPE to~18% clones at 40 μM CAPE (1 week), and <5% clones at 40 μM CAPE (4 weeks in agar) (b). Clones of ≥50 cells were counted as one clone at 1 and 4 weeks in soft agar. A statistically significant difference was seen as early as with a low dose of 5 μM CAPE (1 week (*p = 0.01); and 4 weeks (**p < 0.05). The experimental differences were determined by two-tailed Student’s t-test; n = 3 and p ≤ 0.05 was taken as a significant difference
Effect of CAPE on MMS apoptosis (Fig. 4)
3 × 104 single cells derived from MMS were cultured and treated with CAPE for 4.5 days, then incubated with Annexin V-FITC and propidium iodide (PI) as described in the Materials and Methods. After 4.5 days, CAPE did not cause apoptosis of bCSCs at the CAPE concentrations tested (CAPE 20 μM and 40 μΜ).
CAPE effects on cell cycle of MMS (Fig. 5)
3 × 104 single cells derived from MMS were treated with 40 μM CAPE or without CAPE for 48 h and cell cycle progression was determined using flow cytometry. MMS were noted to significantly progress from a typical quiescent cell cycle state in G0/G1 (82%), S phase (12%) to a cycling state with an increase in S phase (41%) and subsequent decrease in G0/G1 (54%).
Changes in MMS phenotype by CAPE (Fig. 6)
Single cells derived from MMS were seeded, cultured and treated with 40 μM CAPE for 4.5 days as described in Material and Methods before co-staining with fluorochrome-conjugated monoclonal antibodies against human CD44 (APC) and CD24 (PE). CAPE treatment decreased CD44 levels by ~95%, while another cell population containing 10 to >100-fold lower CD44 content concurrently increased.
CAPE results in loss of bCSC phenotype: Incubation with 40 μM CAPE for 4.5 days causes an ~20–25% decline in CD44 density at about the same CD24 levels as those of newly formed populations due to the MMS+CAPE interaction (a) and ~95% decline in CD44 levels (comparing the green vs. blue trace) with concurrent appearance of different cell populations characterized by 10 to ≥100 times lower CD44 density (blue trace) (b)
Discussion
We have previously shown that CAPE inhibits growth of breast cancer cells, both the estrogen receptor positive as well as the estrogen receptor negative (TNBC) subtypes without significant cytotoxic effect on immortalized, but non-tumorigenic breast cancer cells [36]. Here we show that CAPE inhibits growth of breast cancer stem cells (bCSC) isolated from the aggressive triple negative breast cancer cell line, MDA-231. bCSC were successfully isolated from both the MDA-231 cell line and MDA-231 cell induced nude mouse xenografts. These cells were propagated in culture in the absence of serum (Fig. 1a) and grow as mammospheres (MMS), and when dissociated into single cells, formed MMS again. This process, considered a measure of self-renewal, a CSC trait known to be responsible for regenerating tumor cell mass and cancer recurrence, was repeated for many generations for 4 weeks. Further, we demonstrated that the isolated MMS possessed the identifying characteristics of the CD44+/CD24-/low phenotype, as assessed by flow cytometry (Fig. 1b). The MMS population obtained from 4 weeks of self renewal demonstrated ~75% enrichment of the CD44+/CD24-/low CSC phenotype, (Fig. 1b).
Cancer stem cells are thought to be more resistant to chemo- and radiotherapy than non-CSC tumor cells [40], therefore, agents that interfere with CSC growth could be potentially highly desirable therapeutic agents. We tested CAPE’s effect on MMS self renewal in vitro after incubation with CAPE using different doses (0–40 μM). Self renewal in the absence of serum was inhibited in a dose-dependent fashion, with a 97% inhibition at the highest dose of 40 μM CAPE (Fig. 2a). Further, the ability to form progenitors in the presence of serum was noted to be cell concentration-dependent in the absence of CAPE, while the inhibitory effect after pretreatment with CAPE on progenitor formation was highest at the cell density of 800 cells/well with about 53% inhibition at 20 μM CAPE (Fig. 2b). We next examined whether the inhibitory effect of CAPE on progenitor formation could be recapitulated on soft agar, typically a measure of malignant potential. We observed that pretreatment of dissociated MMS with CAPE at 40 μM resulted in the inhibition of progenitor formation and clonal growth in soft agar (Fig. 3a). In addition, this inhibitory effect was CAPE dose-dependent with the highest inhibition at 40 μM CAPE, an effect that was more pronounced after 4 weeks on soft agar. These results collectively suggest that CAPE inhibits breast cancer stem cell self renewal and progenitor formation with associated inhibitory changes in their inherent malignant potential, suggesting that CAPE could be a valuable therapeutic agent.
We next sought to determine the basis of this inhibitory effect of CAPE on MMS growth. First, we examined the role of apoptosis in CAPE-induced MMS inhibition. CAPE treatment did not cause MMS apoptosis as determined by Annexin V/propodium iodide staining (Fig. 4), lending support to the idea of resistance of CSC to apoptosis from therapeutic agents. However, CAPE induced a number of changes in single cells derived from MMS. bCSC isolated from human tumors are predominantly (75%) in G0/G1 phases [1]. Cell cycle analysis revealed that CAPE-treated bCSC significantly progressed from a quiescent cell cycle state in G0/G1 (82%), S phase (12%) to a cycling state with an increase in S phase (41%) and subsequent decrease in G0/G1 (54%) (Fig. 5). This apparent contradiction of findings can be explained by the view that MMS are usually resistant to killing and cycle very infrequently but, when they are differentiated to progenitors, cells that cycle, they become more sensitive to growth inhibition by CAPE. Under the conditions of our experiments using a CAPE concentration of 40 μM and defined time of treatment, a great majority, but not all cell growth is inhibited. Hence, some differentiated cells are still cycling as shown by flow cytometry. Actually, differentiation of MMS to progeny and cell cycling of differentiated cells might be a prerequisite to the CAPE-mediated observed growth inhibition.
Next, we examined the effects of CAPE on CD44/CD24 expression. Interestingly, we noted changes in the CSC surface marker characteristics, specifically, CD44, where CAPE treatment for 4.5 days of dissociated MMS caused 10 to ≥100-fold decrease in CD44 levels, with only ~5% of high CD44 cell population remaining (Fig. 6). CAPE-mediated decrease in the levels of CD44, a receptor important in tumor growth, supports our view described above that MMS differentiation to progeny and cell cycling of differentiated cells might be a prerequisite to the CAPE-induced observed growth inhibition. CD44 supports anchorage-independent growth in vitro and tumor growth and metastasis in experimental models of solid cancers [41–44]. CD44 interacts with Hyaluronan (HA), a major glycosaminoglycan in the extracellular matrix whose expression is tightly linked to multidrug resistance and tumor progression [45]. Investigations into HA-induced interaction between CD44 (an HA receptor) and Nanog (transcription factor that promotes self renewal and maintenance of pluripotency in embryonic stem cells) in both human breast tumor and human ovarian tumor cells reveal that HA binding to these tumor cells promotes Nanog protein association with CD44, followed by Nanog activation and the expression of pluripotent stem cell regulators [45]. Taken together, targeting HA/CD44-mediated Nanog signaling pathways and ankyrin/cytoskeleton function may represent a novel approach to overcome chemotherapy resistance in breast cancer cells displaying stem cell marker properties during tumor progression.
Our flow cytometry data indicate that in addition to the main MMS signal, ~20–25% of bCSC exhibited substantial decline in CD44 density at about the same CD44 levels as those of newly formed populations due to the MMS+CAPE interaction, and about the same CD24 levels, as those induced by CAPE (Fig. 6a). Further, bCSC incubation with 40 μM CAPE for 4.5 days causes ~95% decline in CD44 levels from that normally present in bCSCs with concurrent appearance of different cell populations characterized by 10 to ≥100 times lower CD44 density (Fig. 6b). Notably, it appears that even when MMS are analyzed by flow cytometry shortly after harvesting, other populations are already evident. Since the 4.5 day incubation of MMS+CAPE dose-dependently decreased soft agar growth (Fig. 3b), with 40 μM CAPE inhibiting it by ~95%, it is likely that CD44 decline or a consequence of that decline could be in part responsible for the inhibition of soft agar growth, a standard measure of a malignant potential. It is also possible that effects of CAPE on gene/protein expression and on cell differentiation could have contributed to this phenomenon. Furthermore, HA-CD44 interaction induces ankyrin (a cytoskeletal protein) binding to MDR1 resulting in the efflux of chemotherapeutic drugs (e.g., doxorubicin and Taxol) and chemoresistance in these tumor cells [45]. Thus, the CAPE-induced decline in CD44 could inhibit binding to MDR1 and the resultant efflux of chemotherapeutic drugs with a subsequent decrease in chemoresistance of the bCSC. Additionally, we have previously found that CAPE causes downregulation of the mdr gene in MDA-231 cells, while a combination of CAPE and Taxol in vitro and in vivo was more effective than either of them alone (not shown), suggesting that CAPE can inhibit efflux of Taxol and be used as an adjuvant to enhance the therapeutic outcome [36] (Omene C et al., manuscript in preparation).
To our knowledge, this is the first report of CAPE’s effects on cancer stem cells, which we show using the breast cancer model. Collectively, our results suggest that CAPE causes pronounced changes in breast cancer stem cell characteristics manifested by inhibition of self renewal, inhibition of clonal expansion in soft agar, and decrease in CD44 content, all signs of decreased potential for malignancy. Further, CAPE induces an increase in the cycling state of the bCSCs, potentially making them more susceptible to chemotherapeutic agents if used in combination. Our results strongly suggest that the MDA-231-derived bCSC are induced into a less malignant state after CAPE treatment and may terminally differentiate their progeny making them more susceptible to chemotherapy. Ongoing research should reveal the effect of CAPE on bCSC from other subtypes of breast cancer.
Abbreviations
- bCSC:
-
Breast cancer stem cells
- CAPE:
-
Caffeic acid phenethyl ester
- HA:
-
Hyaluronan
- LB-15mm:
-
Leibowitz-15 medium for MMS growth (B57+EGF no serum)
- MMS:
-
Mammospheres
- MTT:
-
[3-(4 5-dimethylthiazolyl-2)-2, 5-diphenyl tetrazolium bromide]
- TNBC:
-
Triple negative breast cancer
- ULA:
-
Ultra low attachment
References
Al-Hajj M, Wicha SM, Benito-Hernandez A, Morrison SJ, Mf C (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100:3983–3988
Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65:10946–10951
O’Brien CA, Kresco DJE (2009) Cancer stem cells in solid tumors: an overview. Semin Radiat Oncol 19(2):71–77
Ricci-Vitiani L, Lonbardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon cancer-initiating cells. Nature 445:111–115
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Dontu G, Wicha MS (2005) Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia 10:75–86
Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev 17:1253–1270
Fillmore CM, Kuperwasser C (2008) Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 10(2):1–13
Platt VM, Szoska FC (2008) Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm 5(4):474–486
Pilarski LM, Masellis-Smith A, Belch AR, Yang B, Savani RC, Turley EA (1994) RHAMM, a receptor for hyaluronan-mediated motility, on normal human lymphocytes, thymocytes and malignant B cells: a mediator in B cell malignancy? Leuk Lymphoma 14:363–374
Bourguignon LY, Zhu H, Shao L, Chen YW (2000) CD44 interaction with tiam1 promotes Rac1 signaling and hyaluronic acid-mediated breast tumor cell migration. J Biol Chem 275:1829–1838
Bourguignon LY, Singleton PA, Zhu H, Diedrich F (2003) Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. J Biol Chem 278:29420–29434
Bourguignon LY, Peyrollier K, Gilad E, Brightman A (2007) Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. J Biol Chem 282:1265–1280
Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y (2002) CD44 in cancer. Crit Rev Clin Lab Sci 39:527–579
Akima K, Ito H, Iwata Y, Matsuo K, Watari N, Yanagi M, Hagi H, Oshima K, Yagita A, Atomi Y, Tatekawa I (1996) Evaluation of antitumor activities of hyaluronate binding antitumor drugs: synthesis, characterization and antitumor activity. J Drug Target 4:1–8
Park JI, Cao L, Platt VM, Huang Z, Stull RA, Dy EE, Sperinde JJ, Yokoyama JS, Szoka FC (2009) Antitumor therapy mediated by 5-fluorocytosine and a recombinant fusion protein containing TSG-6 hyaluronan binding domain and yeast cytosine deaminase. Mol Pharm 6(3):801–812
Sy MS, Guo YJ, Stamenkovic I (1992) Inhibition of tumor growth in vivo with a soluble CD44-immunoglobulin fusion protein. J Exp Med 176:623–627
Son S, Lewis BA (2002) Free radical scavenging and antioxidative activity of caffeic acid amide and ester analogues: structure-activity relationship. J Agric Food Chem 50:468–472
Koltuksuz U, Mutus HM, Kutlu R, Ozyurt H, Cetin S, Karaman A, Gurbuz N, Akyol O, Aydin NE (2001) Effects of caffeic acid phenethyl ester and epidermal growth factor on the development of caustic esophageal stricture in rats. J Pediatr Surg 36:1504–1509
Michaluart P, Masferrer JL, Carothers AM, Subbaramaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ (1999) Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 59:2347–2352
Borrelli F, Izzo AA, Di Carlo G, Maffia P, Russo A, Maiello FM, Capasso F, Mascolo N (2002) Effect of a propolis extract and caffeic acid phenethyl ester on formation of aberrant crypt foci and tumors in the rat colon. Fitoterapia 73:S38–S43
Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB (1996) Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA 93:9090–9095
Watabe M, Hishikawa K, Takayanagi A, Shimizu N, Nakaki T (2004) Caffeic acid phenethyl ester induces apoptosis by inhibition of NF-κB and activation of Fas in human breast cancer MCF-7 cells. J Biol Chem 279:6017–6026
Fitzpatrick LR, Wang J, Le T (2001) Caffeic acid phenethyl ester, an inhibitor of nuclear factor-κB, attenuates bacterial peptidoglycan polysaccharide-induced colitis in rats. J Pharmacol Exp Ther 299:915–920
Xiang D, Wang D, He Y, Xie J, Zhong Z, Li Z, Xie J (2006) Caffeic acid phenethyl ester induces growth arrest and apoptosis of colon cancer cells via the beta-catenin/T-cell factor signaling. Anti-Cancer Drugs 17(7):753–762
Chen MF, Keng PC, Lin PY, Yang CT, Liao SK, Chen WC (2004) Cell killing and radiosensitization by caffeic acid phenethyl ester (CAPE) in lung cancer cells. J Radiat Res 45(2):253–260
Guarini L, Su ZZ, Zucker S, Lin J, Grunberger D, Fisher PB (1992) Growth inhibition and modulation of antigenic phenotype in human melanoma and glioblastoma multiforme cells by caffeic acid phenethyl ester (CAPE). Cell Mol Biol 38(5):513–527
Kuo HS, Kuo WH, Lee YJ, Lin WL, Chou FP, Tseng TH (2006) Inhibitory effect of caffeic acid phenethyl ester on the growth of C6 glioma cells in vitro and in vivo. Cancer Letters 28; 234(2):199–208
Chen MJ, Chang WH, Lin CC, Liu CY, Wang TE, Chu CH, Shih SC, Chen YJ (2008) Caffeic acid phenethyl ester induces apoptosis of human pancreatic cancer cells involving caspase and mitochondrial dysfunction. Pancreatology 8(6):566–576
Wu CS, Chen MF, Lee IL, Tung SY (2007) Predictive role of nuclear factor-kappa B activity in gastric cancer: a promising adjuvant approach with caffeic acid phenethyl ester. J Clin Gastroenterol 41(10):871–873
Onori P, DeMorrow S, Gaudio E, Franchitto A, Mancinelli R, Venter J et al (2009) Caffeic acid phenethyl ester decreases cholangiocarcinoma growth by inhibition of NF-kappaB and induction of apoptosis. Int J Cancer 125(3):565–576
Lee KW, Kang NJ, Kim JH, Lee KM et al (2008) Caffeic acid phenethyl ester inhibits invasion and expression of matrix metalloproteinase in SK-Hep1 human hepatocellular carcinoma cells by targeting nuclear factor kappaB. Genes Nutr 2(4):319–322
Yang C, Wu J, Zhang R, Zhang P, Eckard J, Yusuf R, Huang X, Rossman TG, Frenkel K (2005) Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology 15;213(1–2):81–96
Frenkel K, Wei H, Bhimani R, Ye J, Zadunaisky JA, Huang MT, Ferraro T, Conney AH, Grunberger D (1993) Inhibition of tumor promoter-mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester. Cancer Res. 15;53(6):1255–61
Wu J, Horton L, Bosland M, Karkoszka J, Frenkel K (2007) Caffeic Acid Phenethyl Ester (CAPE) as a preventive agent in a preclinical model of breast cancer. Proceedings of the American Association for Cancer Research, Abstract # 4202
Wu J, Omene C, Karkaszka J, Bosland M, Eckard J, Klein CB, Frenkel K (2011) caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in preclinical models of human breast cancer. Cancer Lett. doi:10.1016/j.canlet.2011.04.012
Wu J, Omene C, Karkoszka J, Klein CB, Smith J, Frenkel K (2009) CAPE, a honeybee product, inhibits growth of human breast cancer xenografts by oral and topical routes and suppresses self-renewal of breast cancer stem cells. Proceedings of the American Association for Cancer Research. Abstract. # 2324
Wu J, Omene C, Smith J, Frenkel K (2010) Inhibition of breast cancer stem cells (CSC) self-renewal and growth by CAPE, a product of propolis. Proceedings of the American Association for Cancer Research, Abstract. # 3555
Omene C, Wu J, Qu S, Smith J, Frenkel K (2011) CAPE-induced Inhibition of Breast Cancer Stem Cells (CSC) Self-renewal and Growth by Differentiation to a less Malignant Phenotype, Proceedings of the American Association for Cancer Research, Abstract # 3344
Velasco-Velasquez M, Yu Z, Jiao X, Pestell R (2009) Cancer stem cells and the cell cycle: targeting the drive behind breast cancer. Expert Rev Anticancer Ther 9(3):275–279
Barbour AP, Reeder JA, Walsh MD, Fawcett J, Antalis TM, Gotley DC (2003) Expression of the CD44v2–10 isoform confers a metastatic phenotype: importance of the heparan sulfate attachment site CD44v3. Cancer Res 63:887–892
Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW (2002) Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res 62:2281–2286
Yu Q, Toole BP, Stamenkovic I (1997) Induction of apoptosis of metastatic mammary carcinoma cells in vivo by disruption of tumor cell surface CD44 function. J Exp Med 186:1985–1996
Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q (2008) CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res 1;14(21):6751–60
Bourguignon LY, Peyrollier K, Xia W, Gilad E (2008) Hyaluronan-CD44 interaction activates stem cell marker Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated multidrug efflux in breast and ovarian tumor cells. J Biol Chem 20;283(25):17635–51
Aknowledgements
[This work was supported in part by grants BCTR0600476 & ES00260]
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Coral O. Omene and Jing Wu contributed equally to the paper
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Omene, C.O., Wu, J. & Frenkel, K. Caffeic Acid Phenethyl Ester (CAPE) derived from propolis, a honeybee product, inhibits growth of breast cancer stem cells. Invest New Drugs 30, 1279–1288 (2012). https://doi.org/10.1007/s10637-011-9667-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9667-8