Summary
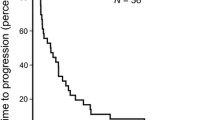
Hepatocellular carcinoma (HCC) remains a lethal treatment-resistant cancer with a median survival of <6 months in patients not considered candidates for radical surgical treatments. SB-715992 is a novel cytotoxic agent implicated in the inhibition of mitotic kinesin spindle protein (KSP). Based on evidence from preclinical models and phase I trials, we conducted a phase II trial of SB-715992 in chemo-naïve patients with advanced HCC. A non-randomized, non-blinded multicentre two-stage phase II study was completed examining the efficacy, toxicity, and pharmacokinetics of SB-715992 at 18 mg/m2 IV q 3 weeks, in patients with measurable locally advanced, metastatic or recurrent HCC. The predictive value of KSP in archival tumour was assessed. Fifteen patients with metastatic HCC received a median of 3 cycles of SB-715992. The most common grade 3+ toxicities were granulocytopenia, leukocytopenia, diarrhea and liver transaminase rise. Overall confirmed response rate was 0%. Seven (46%) patients had a best response of stable disease at the 8-week evaluation (median duration 3.9 months) Median time to progression was 1.61 months (95%CI = 1.31–3.94 months) SB-715992 plasma concentrations were comparable to those observed in the phase I studies. Expression of KSP by immunohistochemistry was observed in only four of eight evaluable samples with strong expression reported in only two. No correlation was observed between intensity of KSP staining and clinical outcome. Among these patients with preserved hepatic function and good performance status, SB-715992 was generally well tolerated. However, no conclusive evidence of benefit was seen with SB-715992 monotherapy in HCC.




Similar content being viewed by others
References
Parkin DM, Bray F, Ferlay J et al (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
El-Serag HB, Mason AC (1999) Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med 340(10):745–750
Perry JF, Strasser SI et al (2003) Pharmacotherapy of hepatocellular carcinoma. Expert Opin Pharmacother 4(12):2175–2185
Bismuth H, Majno PE et al (1999) Liver transplantation for hepatocellular carcinoma. Semin Liver Dis 19(3):311–322
Poon RT, Fan ST et al (2000) Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol 18(5):1094–1101
Camma C, Schepis F et al (2002) Transarterial chemoembolization for unresectable hepatocellular carcinoma: metaanalysis of randomized controlled trials. Radiology 224(1):47–54
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37(2):429–442
Lai CL, Wu PC et al (1988) Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer 62(3):479–483
Johnson PJ (2003) Are there indications for chemotherapy in hepatocellular carcinoma? Surg Oncol Clin N Am 12(1):127–134
Yeo W, Zee B, Leung WT et al (2004) A phase III study of doxorubicin (A) versus cisplatin (P)/ interferonA-2B (I)/ doxorubicin (A)/fluorouracil (F) combination chemotherapy (PIAF) for inoperable hepatocellular carcinoma (HCC). Proc Am Soc Clin Oncol 22:2004 (abstr 4026)
Llovet J, Ricci S, Mazzaferro V, Hilgard P, Raoul J, Zeuzem S, Poulin-Costello M, Moscovici M, Voliotis D, Bruix J, for the SHARP Investigators (2007) Sorafenib improves survival in advanced Hepatocellular Carcinoma (HCC): results of a Phase III randomized placebo-controlled trial. J Clin Oncol 25:abstr LBA1
Llovet JM, Bruix J (2000) Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 32(3):679–680 (letter)
Wood KW, Cornwell WD, Jackson JR (2001) Past and future of the mitotic spindle as an oncology target. Curr Opin Pharmacol 1:370–377
Lee Y, Jia Z, Sakowicz R (2002) Inhibitors of the mitotic kinesin KSP: biochemical mechanism of action. Proc Am Assoc Cancer Res 43:A325
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288:88–95
Chu QS, Holen KD, Rowinsky EK, Wilding G, Volkman JL, Orr JB, Williams DD, Hodge JP, Sabry J (2004) Phase I trial of novel kinesin spindle protein (KSP) inhibitor SB-715992 IV Q 21 days. Proc Am Soc Clin Oncol 22:A2078
Heath EI, Alouisi A, Eder JP, Valdivieso M, Vasist LS, Appleman L, Bhargava P, Colevas AD, Lorusso PM, Shapiro G (2006) A phase I dose escalation trial of ispinesib (SB-715992) administered days 1–3 of a 21-day cycle in patients with advanced solid tumors. Proc Am Soc Clin Oncol 24:A2026
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oostrom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors (RECIST guidelines). J Natl Cancer Inst 92:205–216
Huang M, Liu G (1999) The study of innate drug resistance of human hepatocellular carcinoma Bel7402 cell line. Cancer Lett 135:97–105
Kuo MT, Zhao JY, Teeter LD et al (1992) Activation of multidrug resistance (P-glycoprotein) mdr3/mdr1a gene during the development of hepatocellular carcinoma in hepatitis B virus transgenic mice. Cell Growth Differ 3:531–540
Johnson RK, McCabe FL, Caulder E et al (2002) SB-715992, a potent and selective inhibitor of the mitotic kinesin KSP, demonstrates broad-spectrum activity in advanced murine tumors and human tumor xenografts. Proc Am Assoc Cancer Res 43:A1335
Acknowledgement
The National Cancer Institute of Canada Clinical Trials Group supported the design, conduct, evaluation and manuscript preparation of this trial; drug was supplied by the National Cancer Institute Division of Cancer Therapy Evaluation Program (CTEP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Knox and Sharlene are co-principal investigators.
Rights and permissions
About this article
Cite this article
Knox, J.J., Gill, S., Synold, T.W. et al. A phase II and pharmacokinetic study of SB-715992, in patients with metastatic hepatocellular carcinoma: a study of the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG IND.168). Invest New Drugs 26, 265–272 (2008). https://doi.org/10.1007/s10637-007-9103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9103-2