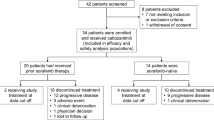
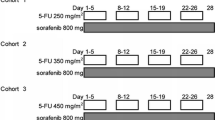
Summary
Navitoclax (ABT-263) is an oral BCL2 homology-3 mimetic that binds with high affinity to pro-survival BCL2 proteins, resulting in apoptosis. Sorafenib, an oral multi kinase inhibitor also promotes apoptosis and inhibits tumor angiogenesis. The efficacy of either agent alone is limited; however, preclinical studies demonstrate synergy with the combination of navitoclax and sorafenib. In this phase 1 study, we evaluated the combination of navitoclax and sorafenib in a dose escalation cohort of patients with refractory solid tumors, with an expansion cohort in hepatocellular carcinoma (HCC). Maximum tolerated dose (MTD) was determined using the continual reassessment method. Navitoclax and sorafenib were administered continuously on days 1 through 21 of 21-day cycles. Ten patients were enrolled in the dose escalation cohort and 15 HCC patients were enrolled in the expansion cohort. Two dose levels were tested, and the MTD was navitoclax 150 mg daily plus sorafenib 400 mg twice daily. Among all patients, the most common grade 3 toxicity was thrombocytopenia (5 patients, 20%): there were no grade 4 or 5 toxicities. Patients received a median of 2 cycles (range 1–36 cycles) and all patients were off study treatment at data cut off. Six patients in the expansion cohort had stable disease, and there were no partial or complete responses. Drug-drug interaction between navitoclax and sorafenib was not observed. The combination of navitoclax and sorafenib did not increase induction of apoptosis compared with navitoclax alone. Navitoclax plus sorafenib is tolerable but showed limited efficacy in the HCC expansion cohort. These findings do not support further development of this combination for the treatment of advanced HCC. This phase I trial was conducted under ClinicalTrials.gov registry number NCT01364051.

Similar content being viewed by others
Data availability
Data is available upon request.
References
Kale J et al (2018) Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep 19(9)
Tse C et al (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68(9):3421–3428
Harrison CN et al (2022) Addition of Navitoclax to Ongoing Ruxolitinib Therapy for Patients With Myelofibrosis With Progression or Suboptimal Response: Phase II Safety and Efficacy. J Clin Oncol 40(15):1671–1680
Pullarkat VA et al (2021) Venetoclax and Navitoclax in Combination with Chemotherapy in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia and Lymphoblastic Lymphoma. Cancer Discov 11(6):1440–1453
Rudin CM et al (2012) Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res 18(11):3163–3169
Bertino EM et al (2021) Phase IB Study of Osimertinib in Combination with Navitoclax in EGFR-mutant NSCLC Following Resistance to Initial EGFR Therapy (ETCTN 9903). Clin Cancer Res 27(6):1604–1611
Walensky LD (2012) From mitochondrial biology to magic bullet: navitoclax disarms BCL-2 in chronic lymphocytic leukemia. J Clin Oncol 30(5):554–557
Joly F et al (2022) A phase II study of Navitoclax (ABT-263) as single agent in women heavily pretreated for recurrent epithelial ovarian cancer: The MONAVI - GINECO study. Gynecol Oncol 165(1):30–39
Arai S et al (2018) Tyrosine Kinase Inhibitors Increase MCL1 Degradation and in Combination with BCLXL/BCL2 Inhibitors Drive Prostate Cancer Apoptosis. Clin Cancer Res 24(21):5458–5470
Hikita H et al (2010) The Bcl-xL inhibitor, ABT-737, efficiently induces apoptosis and suppresses growth of hepatoma cells in combination with sorafenib. Hepatology 52(4):1310–1321
Liu L et al (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66(24):11851–11858
Watanabe J et al (2002) Bcl-xL overexpression in human hepatocellular carcinoma. Int J Oncol 21(3):515–519
Meng XW et al (2007) Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43–9006)-induced TRAIL sensitization. J Biol Chem 282(41):29831–29846
Busche S et al (2021) BH3-only protein expression determines hepatocellular carcinoma response to sorafenib-based treatment. Cell Death Dis 12(8):736
Zhang W et al (2008) Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 22(4):808–818
Sung H et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249
Gholam PM, Iyer R, Johnson MS (2019) Multidisciplinary Management of Patients with Unresectable Hepatocellular Carcinoma: A Critical Appraisal of Current Evidence. Cancers (Basel) 11(6)
Llovet JM et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Garrett-Mayer E (2006) The continual reassessment method for dose-finding studies: a tutorial. Clin Trials 3(1):57–71
Goodman SN, Zahurak ML, Piantadosi S (1995) Some practical improvements in the continual reassessment method for phase I studies. Stat Med 14(11):1149–1161
National Institutes of Health, National Cancer Institute (2009) Common Terminology criteria for adverse events (CTCAE) version 4.0, D.o.H.a.H. Services, Editor
Eisenhauer EA et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–47
Sorafenib (2018) [Package insert] Whippany, NJ: Bayer HealthCare Pharmaceuticals Inc. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021923s020lbl.pdf
Yu C et al (2005) The Role of Mcl-1 Down-regulation in the Pro-apoptotic Activity of the Raf Kinase Inhibitor BAY 43–9006. Oncogene 24:6861–6869
Bruix J et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66
Kudo M et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173
Abou-Alfa GK et al (2018) Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 379(1):54–63
Zhu AX et al (2019) Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20(2):282–296
Finn RS et al (2020) Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 382(20):1894–1905
Cheng AL et al (2022) Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76(2):862–873
Abou-Alfa GK et al (2022) Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evidence 1(8):EVIDoa2100070
Yau T et al (2020) Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol 6(11):e204564
Tolcher AW et al (2015) Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with irinotecan: results of an open-label, phase 1 study. Cancer Chemother Pharmacol 76(5):1041–1049
Cleary JM et al (2014) A phase I clinical trial of navitoclax, a targeted high-affinity Bcl-2 family inhibitor, in combination with gemcitabine in patients with solid tumors. Invest New Drugs 32(5):937–945
Puglisi M et al (2021) A Phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol 17(21):2747–2758
Vlahovic G et al (2014) A phase I safety and pharmacokinetic study of ABT-263 in combination with carboplatin/paclitaxel in the treatment of patients with solid tumors. Invest New Drugs 32(5):976–984
Tolcher AW et al (2015) Safety, efficacy, and pharmacokinetics of navitoclax (ABT-263) in combination with erlotinib in patients with advanced solid tumors. Cancer Chemother Pharmacol 76(5):1025–1032
Acknowledgements
We acknowledge Dr Rory Smoot for his contribution to the preclinical development of this study; Dr. Andrew Ralya and Dr. Ramy Abdelrahman who contributed to the design of the early pharmacokinetics studies and analysis; and Renee McGovern, for her contribution to the development of the assays for navitoclax and sorafenib.
Funding
Supported by the Mayo Clinic U01 (CA069912), UM1 (CA186686), P30 (CA015083), and Hepatobiliary SPORE (P50 CA210964) grants.
Author information
Authors and Affiliations
Contributions
O.E.- data interpretation, manuscript writing and review; J.Y.- study design, safety monitoring, data analysis and data management, manuscript review; E.K.- data analysis, writing and review of manuscript; J.R.- development of preclinical data, study design, correlative studies, manuscript review; M.E., M.B., Y.L., M.S., E.S., Y.J., A.K., J.P., S.D. were sub-Investigators at study sites, and reviewed the manuscript; N.T.- conception and design of the study, data analysis and interpretation, editing the manuscript and review the data, Final approval of the version to be published, (she was a CRADA PI for the NCI ABT263 program and submitted an IND to the FDA); G.G., L. R., G.S.- study design, manuscript review and approval of submission; A.W.H., S.K. - scientific input in trial rationale, reviewed manuscript; B.C, J.H., and A.A. - Oversight of study conduct, patient enrollment and management, Manuscript writing. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
J. R.- Consulting or Advisory Role for Elucida Oncology. Y.L.- Consulting or Advisory Role for AstraZeneca, Janssen, Lilly, Turning Point Therapeutics; Research Funding to Institution from Merck, Tolero Pharmaceuticals, Blueprint Medicines, Daiichi Sankyo, Genmab, Mirati Therapeutics, Roche/Genentech. M.S.- Honoraria from Horizon CME, Daiichi Sankyo, Deciphera, AADi; Travel, Accommodations, Expenses from Horizon CME. M.E.-Consulting or Advisory Role for WindMIL, Flame Biosciences, Sanofi/Regeneron, Kanaph Therapeutics, Coherus BioSciences, GE Healthcare, BioAtla; Travel, Accommodations, Expenses from Nektar; Research Funding to institution from Bristol-Myers Squibb, Apexigen, Nektar, GlaxoSmithKline, Windmil, United Therapeutics; Patent application for radiopharmaceutical to treat small cell lung cancer by Institution; Stock and Other Ownership Interests in Biomarker Strategies, Creatv MicroTech; Other Relationship: AstraZeneca/MedImmune, Boehringer Ingelheim, Takeda, Seagen, Anheart Therapeutics. A.K.- Consulting or Advisory Role for Bayer Health, Bristol-Myers Squibb, Merck, Exelixis, Genentech/Roche, Eisai, AstraZeneca; Research Funding to institution from Bristol-Myers Squibb, Merck, Bayer/Onyx, Genentech, Adaptimmune, Hengrui Pharmaceutical; Honoraria from Merck, Exelixis, Bayer Health, Bristol-Myers Squibb, Eisai, Genentech/Roche, AstraZeneca; Travel, Accommodations, Expenses from Exelixis, Merck, Bayer/Onyx, Bristol-Myers Squibb, Eisai, Genentech/Roche, AstraZeneca. J.P.- Consulting or Advisory Role for Lilly, Seagen. S.D.- Research Funding to institution from Bristol-Myers Squibb, Merck, Boston Scientific, Symphogen, I-Mab, TriSalus Life Sciences, Tvardi Therapeutics, EMD Serono, ORIC Pharmaceuticals; Stock and Other Ownership Interests in Johnson & Johnson/MedTech. L.R- has received grant support from ARIAD Pharmaceuticals, Bayer, BTG International, Exact Sciences, Gilead Sciences, Glycotest, RedHill, Target PharmaSolutions, and Wako Diagnostics; he has provided advisory services to Bayer, Exact Sciences, Gilead Sciences, GRAIL, QED Therapeutics, and TAVEC. G.S.- Consulting or Advisory Role to Bionaut Labs, Ellipses Pharma, Gencirq, Epizyme, Array BioPharma, Apexigen, Oncogenuity, Oncusp Therapeutics, Concarlo, Shanghai Pharma, Astex Pharmaceuticals, January Therapeutics, Sellas Life Sciences, PureTech, AADi, Kirilys Therapeutics, Agenus, Boehringer Ingelheim, Ipsen; Travel, Accommodations, Expenses from Array BioPharma, Epizyme, Boehringer Ingelheim; Patents, Royalties, Other Intellectual Property by institution Companion diagnostics for CD4 inhibitors, Patent granted to develop a new technology called PNAs for cancer therapy; Stock and Other Ownership Interests in GenCirq, Bionaut Labs, January Therapeutics; Research Funding to institution from Astex Pharmaceuticals, Incyte, Calithera Biosciences, Lilly, Daiichi Sankyo, Fortress Biotech, Karyopharm Therapeutics, Oxford BioTherapeutics, Astex Pharmaceuticals, TopAlliance BioSciences Inc, Adaptimmune, SpringWorks Therapeutics, TRACON Pharma. A.WH.- Research Funding to institution from ProLynx, Amgen; Patents, Royalties, Other Intellectual Property by institution in TORL Therapeutics; Consulting or Advisory Role for Oxcia; Speakers' Bureau for OncLive. S.K.- Consulting or Advisory Role for Attivare Therapeutics; Research Funding to Institution from Takeda; Patents, Royalties, Other Intellectual Property owned by Mayo Clinic an antibody developed in the Kaufmann laboratory; Uncompensated Relationships with ProLynx. A.A.- Consulting or Advisory Role for Janssen, EMD Serono, Zai Lab, Swiss Rockets, Mirati Therapeutics; Research Funding to institution from Bayer Schering Pharma, Tesaro, I-Mab, Kura Oncology, Merck KGaA, Zai Lab, Jacobio; Uncompensated Relationships with Merck KGaA, Zai Lab, Swiss Rockets. J.H.- reports advisory funds from Bayer, Merck, BeiGene, Incyte, Taiho; and research funding to institution from Merck, Treos Bio, Senhwa pharmaceuticals, Bayer, Incyte, TriOncology, Seattle Genetics, Hutchison MediPharma, Pionyr Immunotherapeutics, Trovogene, G1 Therapeutics, Roche, Pfizer, Cardiff Oncology, Mirati Therapeutics, Xilis Inc. B.C.- Research Funding to institution from GlaxoSmithKline/Novartis; Speakers' Bureau for Clinical Care Options/NCCN.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emiloju, O.E., Yin, J., Koubek, E. et al. Phase 1 trial of navitoclax and sorafenib in patients with relapsed or refractory solid tumors with hepatocellular carcinoma expansion cohort. Invest New Drugs 42, 127–135 (2024). https://doi.org/10.1007/s10637-024-01420-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-024-01420-8