Abstract
Purpose
We evaluate morphological and functional correlations in patients with acute central serous chorioretinopathy (CSC).
Methods
A prospective study was conducted on 50 patients with an acute CSC episode lasting less than 3 months. At baseline, assessments included optical coherence tomography (OCT), best-corrected visual acuity (BCVA), contrast sensitivity (CS), microperimetry (MP), and multifocal electroretinography (mfERG). A correlation analysis between OCT morphological parameters (maximal subretinal fluid height (SRF), central retinal thickness (CRT), and macular volume (MV)) and functional parameters was conducted on the affected eye for each patient.
Results
Among the morphological parameters, SRF showed the strongest correlations with functional parameters (r absolute value range = 0.10–0.70). Weak correlations were observed between BCVA and morphological parameters (r absolute value range = 0.14–0.26). Average retinal sensitivity (MP-A) was the functional parameter displaying the most robust negative correlation with morphological parameters (r absolute value range = 0.61–0.70). In contrast, average contrast sensitivity (CS-A) and mfERG average amplitude density in the first (mfERG-A1) and second (mfERG-A2) ring showed weak to moderate (r absolute value range = 0.35–0.56) yet statistically significantly nonzero correlations.
Conclusions
SRF and CRT could serve as the most representative morphological proxies for visual function deficit in acute CSC patients. Retinal sensitivity, as measured by MP, may be superior to BCVA in clinical research studies or when an in-depth visual function evaluation is needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Central serous chorioretinopathy (CSC) is the fourth most common non-surgical maculopathy, and it is associated with pachychoroid, irregularities in the retinal pigment epithelium (RPE), and the accumulation of subretinal fluid (SRF) [1, 2]. The latest theory on the pathophysiological processes in CSC describes an increase in choroidal venous pressure, leading to the formation of intervortex anastomoses within the macula. This leads to choroidal hyperpermeability and increased interstitial pressure that ultimately results in leakage of fluid through the RPE [3]. CSC usually affects the middle-aged working population and is associated with several risk factors including excessive endogenous or exogenous corticosteroids [1].
In acute CSC, best-corrected visual acuity (BCVA) is usually only mildly impaired [1, 4, 5]. However, more precise functional tests, such as contrast sensitivity (CS), microperimetry (MP), and multifocal electroretinography (mfERG), often reveal more significant visual function impairment [6,7,8,9]. In patients with spontaneous SRF resolution within the first three months, all functional tests improve shortly after SRF resolution, with the exception of CS, which shows a gradual and delayed improvement [5]. A study showed that the maximal SRF height, a parameter which has to be manually measured with optical coherence tomography (OCT), exhibits a strong correlation with visual function parameters and subjective visual function impairment [4].
In acute CSC, where neurosensory retinal morphology is not significantly altered and RPE irregularities are limited to PED [1], visual function impairment occurs within the area of SRF accumulation [5]. In chronic CSC, characterized by the persistence of SRF for at least 6 months, irreversible vision function loss occurs and could lead to legal blindness [10]. A morphological and functional correlation study showed that serous retinal detachment height at the fovea did not correlate with BCVA in chronic CSC, where maximal neurosensory detachment is typically shallower when compared to acute CSC [1, 11]. On the other hand, studies have revealed that in chronic CSC, retinal sensitivity and mfERG parameters correlated negatively with inner/outer segments (IS/OS) line defects [12] and that retinal sensitivity is negatively correlated with RPE changes as observed on fundus autofluorescence (FAF) [13]. Hence, visual function impairment in chronic CSC primarily arises from retinal morphology alterations in the RPE and the outer retinal layers [11].
The aim of this study was to investigate visual function impairment in acute CSC by employing several visual function tests, including BCVA, CS, MP, and mfERG. Furthermore, our objective was to identify which frequently employed OCT morphological parameters (maximal subretinal fluid height (SRF), central retinal thickness (CRT), and macular volume (MV)) best reflect visual function impairment through the assessment of correlations between morphological and functional parameters.
Methods
A prospective study was conducted at the University Eye Clinic in Ljubljana from 2018 to 2021. The study was conducted in accordance with the Declaration of Helsinki and approved by the National Medical Ethics Committee of the Republic of Slovenia (protocol code 0120–141/2018/4). Patients with CSC who met specific inclusion and exclusion criteria and provided informed consent were enrolled. CSC was characterized by the accumulation of SRF in the fovea, with angiographic evidence of leakage on fluorescein angiography (FA) and indocyanine green angiography (ICGA). Inclusion criteria required patients to have an acute CSC episode lasting less than 3 months and to be aged 18 to 65 years. Exclusion criteria ruled out individuals with other macular conditions besides CSC, ocular pathologies that could influence visual function parameters and allergy to fluorescein. Patients underwent a comprehensive ophthalmological examination at baseline, which included slit lamp examination, fundoscopy, and on the same day, they also underwent OCT, FA, ICGA, BCVA, CS, MP, and mfERG assessments.
Multimodal imaging was conducted using the Spectralis ophthalmic imaging platform (Heidelberg Engineering, Inc., Heidelberg, Germany). OCT of the macula was performed with a 30° field of view lens. The imaging platform software automatically determined the CRT within a circular region of 1-mm diameter centered on the fovea and the MV within a region of 3-mm diameter. A retina specialist manually measured the maximal height of SRF within the central 3-mm diameter around the fovea.
BCVA was assessed using the ETDRS chart (Precision Vision, Illinois, USA), with results converted to logMAR values [14]. CS was evaluated using the FACT chart (Stereooptical CO, Illinois, USA) at spatial frequencies of 1.5, 3.0, 6.0, 12.0, and 18.0 cycles per degree (cpd) [15]. The average CS was then calculated and expressed as log contrast sensitivity. Both BCVA and CS measurements were conducted following the standard protocol, with assessments carried out in the affected eye only.
Retinal sensitivity assessment was performed using MP (Nidek Technologies, MP1, 2002, Padua). A 1° red cross was utilized as a fixation target. A radial grid pattern was used to evaluate retinal sensitivity across 45 test points within a 12° area surrounding the foveola [16, 17]. The test employed a 4–2 staircase strategy, with a stimulus projection time of 200 ms and a stimulus size equivalent to Goldmann III [17]. The average retinal sensitivity (MP-A) was derived from all the tested points. Retinal sensitivity within the central 4° surrounding the foveola was assessed using the 13 central test points (MP-C). Paracentral retinal sensitivity, spanning from 4° to 12° away from the foveola, was evaluated based on 32 test points (MP-P).
MfERG measurements were performed following the standards set by the International Society for Clinical Electrophysiology of Vision (ISCEV) [18]. The HK electrode was positioned in the lower conjunctival sac [19], a reference electrode was placed just behind the temporal orbital bone rim, and the grounding electrode was situated on the glabella. A cathode-ray tube (CRT) screen (RETI port, Roland Consult, Germany) was utilized to display the stimulus. The stimulus, designed to elicit responses from the 30° area around the foveola, consisted of alternating black and white hexagonal fields. Stimulation cycles with a duration of 50 s were recorded and repeated eight times to calculate mean amplitude densities and implicit times for the first through fifth rings [20].
To evaluate correlations between morphological and functional parameters in a single eye, Pearson correlation coefficient was used. Statistical analysis was performed using the IBM SPSS Statistics for Windows, version 28 (IBM Corp., Armonk, NY, USA), with a cutoff at p < 0.05 for statistical significance.
Results
Out of 50 patients, the average age was 44.7 (± 9.9), with 43 males and seven females included. The average duration of symptoms at the time of presentation was 1.4 (± 1.3) months. Five patients reported the use of exogenous corticosteroids in various forms (oral, nasal, dermal) at baseline. One patient used oral steroids for systemic lupus erythematosus, two patients used nasal steroids for allergic rhinitis, and two other patients used dermal steroids for psoriasis.
At baseline, CRT, MV, and SRF had average values (± SD) of 453 (± 133) µm, 3.04 (± 0.71) mm3, and 216 (± 144) µm, respectively. The BCVA was on average 0.19 (± 0.15); the CS 1.5 cpd, 3.0 cpd, 6.0 cpd, 12.0 cpd, 18.0 cpd, and the overall CS averages were 1.43 (± 0.24), 1.48 (± 0.29), 1.16 (± 0.59), 0.57 (± 0.63), 0.20 (± 0.35), and 0.97 (± 0.33), respectively. Retinal sensitivity averaged 9.0 (± 5.3) dB in the central region (MP-C), 12.6 (± 5.2) dB in the paracentral region (MP-P), and 11.6 (± 5.1) dB overall (MP-A). For multifocal ERG amplitude measurements, the average values from first to fifth ring were 58.7 (± 22.5) nV/deg2, 37.7 (± 11.3) nV/deg2, 25.4 (± 5.1) nV/deg2, 17.2 (± 3.1) nV/deg2 and 13.2 (± 2.7) nV/deg2, respectively. Latency measurements in mfERG from first to fifth ring averaged 42.3 (± 5.3) ms, 39.3 (± 1.9) ms, 37.4 (± 1.0) ms, 36.9 (± 1.0) ms, and 37.4 (± 1.1) ms, respectively. Comparison of functional parameters for healthy subjects and our acute CSC cohort is presented in Table 1. Functional parameters in healthy subjects were sourced from the available literature. BCVA in healthy eyes was obtained from 19 patients aged 45–49 [21]; CS in healthy eyes was measured in 27 patients with an average age of 38.8 [22]; MP-A measurements in normal controls were obtained from 33 patients aged 40–49 [16]; mfERG normative values from the University Eye Clinic in Ljubljana were obtained from 20 healthy individuals, which were not age matched to our CSC cohort.
There was a weak correlation between BCVA and CRT (p = 0.32 when testing the null hypothesis of zero correlation) and BCVA and SRF (p = 0.06). On the other hand, correlation analysis showed strong negative correlation between MP-A and CRT (r = -0.61, p < 0.01) and MP-A and SRF (r = -0.70, p < 0.01) (Fig. 1). The correlation heatmap between morphological and functional parameters in our acute CSC patients can be seen in Table 2.
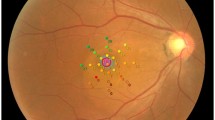
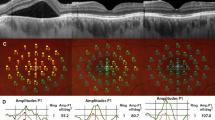
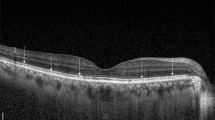
Figure 2 depicts a patient displaying significant SRF accumulation beneath the fovea. Despite this, the BCVA remained excellent (1.0; Snellen). Yet, when subjected to detailed functional tests like MP and mfERG, there was a marked decline in retinal sensitivity and amplitude densities.
Acute central serous chorioretinopathy (CSC) patient with excellent best-corrected visual acuity (BCVA) (A) and severely reduced retinal sensitivity on microperimetry (B) and reduced amplitude densities on multifocal electroretinogram (C). Central retinal thickness (CRT); macular volume (MV); and subretinal fluid (SRF)
Discussion
Our study revealed that all visual function parameters in acute CSC patients were compromised. Among the morphological parameters, maximal SRF exhibited the strongest correlations with functional parameters. Weak correlations were observed between BCVA and morphological parameters. MP-A was the functional parameter that had the strongest negative correlation with morphological parameters. In contrast, CS-A, mfERG-A1, and mfERG-A2 displayed weak to moderate correlations that were statistically significantly different from zero.
When it comes to visual function loss, the mechanisms differ between acute and chronic CSC. In acute CSC, due to the retinal layer morphology being largely intact, visual function impairment is caused by the accumulation of SRF [4, 5, 7, 23]. In contrast, in chronic CSC, visual function loss predominantly arises due to the disruption of the outer retinal layers and RPE [11,12,13]. Rapid resolution of SRF in acute CSC is associated with the normalization of the majority of visual function parameters [5]. In contrast, chronic CSC can lead to irreversible vision loss and legal blindness [10].
In our study, very strong correlations were observed among all morphological parameters. SRF exhibited the strongest correlations between morphological and functional parameters. Gerendas et al. found a stronger correlation between SRF height and retinal sensitivity than between total SRF volume and retinal sensitivity [4]. Moreover, of all morphological parameters, subjective vision impairment handicap correlated most strongly with SRF height [4]. The maximal SRF height appears to be an important morphological parameter in CSC treatment, as patients with lower SRF demonstrated a good response to the subthreshold micropulse laser [24]. Although SRF height appears to be the most indicative morphological parameter for assessing visual function impairment and subjective handicap, it still requires manual measurement within the OCT software.
CRT is a widely used morphological parameter for monitoring the natural course and evaluating treatment effectiveness in all prevalent retinal maculopathies, including CSC [25]. It measures the retinal thickness in the central 1 mm around the fovea and is automatically generated by OCT software. Due to mostly preserved retinal structure in acute CSC, manual segmentation to obtain accurate measurements is usually not necessary. In our study, CRT correlated statistically significantly with CS-A, MP-A, mfERG-A1, and mfERG-A2. The strength of correlation with functional parameters was relatively similar to that with MV but weaker than with SRF. Therefore, due to the simplicity of obtaining measurements, it could be a valuable clinical parameter for evaluating vision impairment in acute CSC patients.
MV measures the macular volume in the 3 mm around the fovea. A larger MV at baseline has been shown to be associated with worse functional outcomes in acute CSC patients who experience rapid SRF resolution within 3 months [5]. Nevertheless, due to less prevalent use in clinical practice and weaker correlations with functional parameters, when compared to SRF, MV might not be the optimal morphological parameter to evaluate functional outcomes in acute CSC patients.
The distribution of SRF in acute CSC patients may play a significant role in visual function impairment. Patients with a broad and shallow accumulation of SRF might experience less visual impairment compared to those with a narrow and elevated SRF accumulation. In the choroid, the choriocapillaris is essential for delivering oxygen and nutrients to the cells of the outer retina [26]. The diffusion of oxygen and nutrients from the choriocapillaris to the outer retinal layers might be more hindered in patients with high SRF accumulation. This could lead to alterations in the outer retinal layers and poorer functional outcomes after the resolution of SRF.
Standard visual function tests evaluate elements like BCVA, CS, perceptions of color, depth, and motion. Each of these attributes represents an aspect of visual function that can influence an individual's overall visual capability [27]. BCVA, which represents the ability to recognize small details precisely, is the most commonly used visual function parameter. However, it only represents one aspect of visual function [27]. In our study, although BCVA was affected in CSC patients, it did not significantly correlate with any of the morphological parameters. Our cohort included only patients with acute CSC, none of whom had significant alterations in the outer retinal layers, such as IS/OS segment line defects or RPE alterations, known to correlate with BCVA worsening in longstanding CSC episodes [11,12,13]. Moreover, some of our patients exhibited excellent BCVA (1.0) yet had impairments in all other visual function tests. Figure 2 shows a patient with extensive SRF accumulation under the fovea; however, BCVA remained excellent (1.0). Nevertheless, more precise functional tests such as MP and mfERG showed severely reduced retinal sensitivity and amplitude densities. This clearly indicates that other functional tests are more sensitive and better determine visual function impairment than BCVA. CS provides a more accurate reflection of visual function in tasks such as face recognition, reading, and driving than BCVA [28]. In our study, CS at higher spatial frequencies were more affected, and average CS showed weak to moderate correlations with morphological parameters. Among the functional parameters, CS-A showed the strongest correlation with BCVA.
MP-A exhibited the strongest correlations with all the morphological parameters in our study. Microperimetry is widely used because it identifies minor changes in visual function that precede the worsening of visual acuity. As a result, it has become a prevalent functional parameter in clinical research studies to monitor the natural course of diseases and treatment effectiveness [29]. The usefulness of microperimetry might extend to biomarkers for spontaneous CSC resolution, with patients with retinal sensitivity of 20 dB or more being inclined toward spontaneous resolution [8]. Microperimetry offers several advantages for use in clinical practice or at least research trials for CSC patients. These include a strong correlation between MP-A and all other morphological and functional parameters, dynamic changes in measurements following morphological changes, high sensitivity to visual impairment, and a relatively quick and straightforward testing process.
In our study, mfERG amplitudes correlated moderately with morphological parameters, while latencies exhibited weak or very weak correlations. Amplitudes in the first two rings of our cohort fell below the normal range, whereas the more peripheral rings remained within the normal range, which is expected, because SRF in the majority of our patients did not extend beyond the second ring. This aligns with the findings of a study that showed mfERG impairment within the area of SRF accumulation [5]. Conversely, several studies have also indicated that mfERG measurements are affected beyond the area of SRF [23, 30, 31].
Although several studies have compared morphological and some functional parameters in CSC [4, 9, 11, 32], our study evaluated morphological parameters and several functional parameters (BCVA, CS, MP, mfERG) in a single study. We identified MP as the functional test that best correlates with morphological parameters in acute CSC and confirmed previous reports that maximal SRF height is most closely associated with visual function impairment [4]. Although conducting a wide range of functional tests in acute CSC patients is neither time nor cost-effective in a routine clinical practice, our study found that maximal SRF, which is easily measurable, offers the best correlation with visual function deficit. Additionally, MP may be the most sensitive and optimal functional test for use in clinical trials.
In conclusion, our study revealed that maximal height of SRF had the strongest correlations with functional parameters, while CRT exhibited moderate correlations. Therefore, these two morphological parameters might be used as a proxy for functional tests in clinical practice. Regarding visual function variables, BCVA showed very weak correlations with morphological parameters, while MP-A exhibited strong correlations. Therefore, in CSC clinical research studies, or when a detailed visual function evaluation is required, microperimetry might be superior to BCVA measurements.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Daruich A, Matet A, Dirani A, Bousquet E, Zhao M, Farman N et al (2015) Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res 48:82–118. https://doi.org/10.1016/j.preteyeres.2015.05.003
Wang M, Munch IC, Hasler PW, Prünte C, Larsen M (2008) Central serous chorioretinopathy. Acta Ophthalmol 86(2):126–145. https://doi.org/10.1111/j.1600-0420.2007.00889.x
Spaide RF, Gemmy Cheung CM, Matsumoto H, Kishi S, Boon CJF, van Dijk EHC et al (2022) Venous overload choroidopathy: a hypothetical framework for central serous chorioretinopathy and allied disorders. Prog Retin Eye Res 86:100973. https://doi.org/10.1016/j.preteyeres.2021.100973
Gerendas BS, Kroisamer JS, Buehl W, Rezar-Dreindl SM, Eibenberger KM, Pablik E et al (2018) Correlation between morphological characteristics in spectral-domain-optical coherence tomography, different functional tests and a patient’s subjective handicap in acute central serous chorioretinopathy. Acta Ophthalmol 96(7):e776–e782. https://doi.org/10.1111/aos.13665
Kiraly P, Smrekar J, Mekjavić PJ (2022) Visual function during and after an acute central serous chorioretinopathy episode. Doc Ophthalmol 145(1):27–35. https://doi.org/10.1007/s10633-022-09875-x
Lourthai P, Bhurayanontachai P (2017) Pattern of contrast sensitivity changes in acute central serous chorioretinopathy. J Ophthalmol 2017:9053932. https://doi.org/10.1155/2017/9053932
Moschos M, Brouzas D, Koutsandrea C, Stefanos B, Loukianou H, Papantonis F et al (2007) Assessment of central serous chorioretinopathy by optical coherence tomography and multifocal electroretinography. Ophthalmologica 221(5):292–298. https://doi.org/10.1159/000104758
Roisman L, Ribeiro JC, Fechine FV, Lavinsky D, Moraes N, Campos M et al (2014) Does microperimetry have a prognostic value in central serous chorioretinopathy? Retina 34(4):713–718. https://doi.org/10.1097/IAE.0b013e3182a323fe
Yip YW, Ngai JW, Fok AC, Lai RY, Li H, Lam DS et al (2010) Correlation between functional and anatomical assessments by multifocal electroretinography and optical coherence tomography in central serous chorioretinopathy. Doc Ophthalmol 120(2):193–200. https://doi.org/10.1007/s10633-010-9213-6
Mrejen S, Balaratnasingam C, Kaden TR, Bottini A, Dansingani K, Bhavsar KV et al (2019) Long-term visual outcomes and causes of vision loss in chronic central serous chorioretinopathy. Ophthalmology 126(4):576–588. https://doi.org/10.1016/j.ophtha.2018.12.048
Sugiura A, Fujino R, Takemiya N, Shimizu K, Matsuura M, Murata H et al (2017) The association between visual function and retinal structure in chronic central serous chorioretinopathy. Sci Rep 7(1):16288. https://doi.org/10.1038/s41598-017-16339-9
Kim SW, Oh J, Huh K (2012) Correlations among various functional and morphological tests in resolved central serous chorioretinopathy. Br J Ophthalmol 96(3):350–355. https://doi.org/10.1136/bjo.2011.204503
Eandi CM, Piccolino FC, Alovisi C, Tridico F, Giacomello D, Grignolo FM (2015) Correlation between fundus autofluorescence and central visual function in chronic central serous chorioretinopathy. Am J Ophthalmol 159(4):652–658. https://doi.org/10.1016/j.ajo.2014.12.023
Kaiser PK (2009) Prospective evaluation of visual acuity assessment: a comparison of snellen versus ETDRS charts in clinical practice (an AOS Thesis). Trans Am Ophthalmol Soc 107:311–324
Hitchcock EM, Dick RB, Krieg EF (2004) Visual contrast sensitivity testing: a comparison of two F A C T test types. Neurotoxicol Teratol 26(2):271–277. https://doi.org/10.1016/j.ntt.2003.10.007
Midena E, Vujosevic S, Cavarzeran F (2010) Normal values for fundus perimetry with the microperimeter MP1. Ophthalmology 117(8):1571–1576. https://doi.org/10.1016/j.ophtha.2009.12.044
Vujosevic S, Midena E, Pilotto E, Radin PP, Chiesa L, Cavarzeran F (2006) Diabetic macular edema: correlation between microperimetry and optical coherence tomography findings. Invest Ophthalmol Vis Sci 47(7):3044–3051. https://doi.org/10.1167/iovs.05-1141
Hoffmann MB, Bach M, Kondo M, Li S, Walker S, Holopigian K et al (2021) ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update). Doc Ophthalmol 142(1):5–16. https://doi.org/10.1007/s10633-020-09812-w
Hawlina M, Konec B (1992) New noncorneal HK-loop electrode for clinical electroretinography. Doc Ophthalmol 81(2):253–259. https://doi.org/10.1007/bf00156014
Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS et al (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124(1):1–13. https://doi.org/10.1007/s10633-011-9296-8
Elliott DB, Yang KC, Whitaker D (1995) Visual acuity changes throughout adulthood in normal, healthy eyes: seeing beyond 6/6. Optom Vis Sci 72(3):186–191. https://doi.org/10.1097/00006324-199503000-00006
Pesudovs K, Hazel CA, Doran RM, Elliott DB (2004) The usefulness of Vistech and FACT contrast sensitivity charts for cataract and refractive surgery outcomes research. Br J Ophthalmol 88(1):11–16. https://doi.org/10.1136/bjo.88.1.11
Hong IH, Chang IB, Jeon GS, Han JR (2022) Evaluation of acute central serous chorioretinopathy using enhanced depth imaging optical coherence tomography and multifocal electroretinography. Ophthalmologica 245(1):25–33. https://doi.org/10.1159/000516097
Kiraly P, Smrekar J, Jaki MP (2022) Morphological parameters predicting subthreshold micropulse laser effectiveness in central serous chorioretinopathy. Lasers Med Sci 37(8):3129–3136. https://doi.org/10.1007/s10103-022-03574-4
van Rijssen TJ, Mohabati D, Dijkman G, Theelen T, de Jong EK, van Dijk EHC et al (2018) Correlation between redefined optical coherence tomography parameters and best-corrected visual acuity in non-resolving central serous chorioretinopathy treated with half-dose photodynamic therapy. PLoS ONE 13(8):e0202549. https://doi.org/10.1371/journal.pone.0202549
Lejoyeux R, Benillouche J, Ong J, Errera MH, Rossi EA, Singh SR et al (2022) Choriocapillaris: fundamentals and advancements. Prog Retin Eye Res 87:100997. https://doi.org/10.1016/j.preteyeres.2021.100997
Bennett CR, Bex PJ, Bauer CM, Merabet LB (2019) The Assessment of Visual Function and Functional Vision. Semin Pediatr Neurol 31:30–40. https://doi.org/10.1016/j.spen.2019.05.006
Fujita K, Matsumoto CS, Mizutani Y, Imamura Y, Tanaka E, Satofuka S et al (2012) Reading performance with different contrast characters in patients with central serous chorioretinopathy. Acta Ophthalmol 90(7):e575–e577. https://doi.org/10.1111/j.1755-3768.2011.02373.x
Yang Y, Dunbar H (2021) Clinical perspectives and trends: microperimetry as a trial endpoint in retinal disease. Ophthalmologica 244(5):418–450. https://doi.org/10.1159/000515148
Chappelow AV, Marmor MF (2000) Multifocal electroretinogram abnormalities persist following resolution of central serous chorioretinopathy. Arch Ophthalmol 118(9):1211–1215. https://doi.org/10.1001/archopht.118.9.1211
Marmor MF, Tan F (1999) Central serous chorioretinopathy: bilateral multifocal electroretinographic abnormalities. Arch Ophthalmol 117(2):184–188. https://doi.org/10.1001/archopht.117.2.184
Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA (2014) Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina 34(4):705–712. https://doi.org/10.1097/iae.0000000000000001
Acknowledgements
None
Funding
The authors acknowledge financial support from the Slovenian Research Agency (ARRS) (research programs P3-0333, P1-0292, and project N1-0083). The supporting source did not have any role in study design, analysis and interpretation of data, writing the report, and the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
P.K. and P.J.M. contributed to the conceptualization; P.K., P.J.M., M.Š.H., and J.S. were involved in the methodology; P.K and J.S. assisted in the formal analysis; P.K. contributed to the investigation; P.J.M. contributed to the resources; P.K. and J.S. were involved in the data curation; P.K. assisted in the writing—original draft preparation; P.K., P.J.M., M.Š.H., and J.S. contributed to the writing—review and editing; P.K. and M.Š.H. were involved in the visualization; P.J.M. assisted in the supervision; P.K. contributed to the project administration; and P.J.M. was involved in the funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the National Medical Ethics Committee of the Republic of Slovenia (protocol code 0120–141/2018/4) on 13 March 2018.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
The consent for publication has been obtained from all authors.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiraly, P., Šuštar Habjan, M., Smrekar, J. et al. Morphological and Functional Correlations in Acute Central Serous Chorioretinopathy. Doc Ophthalmol 148, 145–153 (2024). https://doi.org/10.1007/s10633-024-09969-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-024-09969-8