Abstract
Purpose
To investigate the functional and structural biomarkers and their correlation with Usher syndrome (USH).
Methods
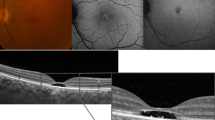
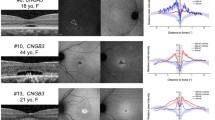
Medical records, imaging and electrophysiology test results of USH patients attending the Save Sight Institute between 2012 and 2017 were reviewed. Best corrected visual acuity (BCVA), ultra-widefield autofluorescence (UW-FAF), spectral-domain optical coherence tomography (SD-OCT), full-field electroretinogram and pattern electroretinogram (pERG) were performed. SD-OCT scans assessed central macular thickness (CMT), greatest linear diameter of preserved outer retinal layers—macular island (MI) and presence of cystoid macular edema (CME). UW-FAF images were qualitatively graded to identify hypo/hyperfluorescence patterns in the peripheral fundus.
Results
Thirty-six eyes from 18 subjects were included. Mean BCVA was 0.22 ± 0.3 LogMAR. MI extent was significantly associated with better vision (β = − 0.175 per 1000 µm; R2 = 0.487; P = 0.002; Fig. 4). A higher pERG P50 was associated with a larger macular island (β = 782 per µV; R2 = 0.238; P = 0.025), while a higher pERG N95 was associated with a smaller macular island (β = − 499 per µV; R2 = 0.219; P = 0.030). Mean CMT was 271 ± 35 μm and was significantly associated with better vision (β = − 0.083 per 10 µm; R2 = 0.612; P < 0.001). CME was diagnosed in 47.2% (n = 17) eyes. There was no significant difference in mean BCVA for those with CME (0.19 ± 0.2 LogMAR) and without CME (0.40 ± 0.5; R2 = 0.081; P = 0.17). All patients had abnormal UW-FAF. Four main patterns of change were identified (granular 55%, annular 11%, bone spicule 17% and patchy 17%). Patients with the patchy pattern demonstrated worse BCVA in comparison with those with granular (P < 0.0001) and bone spicule (P = 0.0179) patterns.
Conclusions
Structural changes identified on OCT and UW-FAF correlated with BCVA and pERG in this cohort representing different stages of the disease. These parameters could represent reliable biomarkers in therapeutic clinical trials on USH.








Similar content being viewed by others
References
Millan JM, Aller E, Jaijo T, Blanco-Kelly F, Gimenez-Pardo A, Ayuso C (2011) An update on the genetics of usher syndrome. J Ophthalmol 2011:417217
Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368:1795–1809
Mitamura Y, Mitamura-Aizawa S, Nagasawa T, Katome T, Eguchi H, Naito T (2012) Diagnostic imaging in patients with retinitis pigmentosa. J Med Investig 59:1–11
Strong S, Liew G, Michaelides M (2017) Retinitis pigmentosa-associated cystoid macular oedema: pathogenesis and avenues of intervention. Br J Ophthalmol 101:31–37
Sliesoraityte I, Peto T, Mohand-Said S, Sahel JA (2015) Novel grading system for quantification of cystic macular lesions in Usher syndrome. Orphanet J Rare Dis 10:157
Walia S, Fishman GA, Hajali M (2009) Prevalence of cystic macular lesions in patients with Usher II syndrome. Eye 23:1206–1209
Triolo G, Pierro L, Parodi MB, De Benedetto U, Gagliardi M, Manitto MP, Bandello F (2013) Spectral domain optical coherence tomography findings in patients with retinitis pigmentosa. Ophthalmic Res 50:160–164
Witkin AJ, Ko TH, Fujimoto JG, Chan A, Drexler W, Schuman JS, Reichel E, Duker JS (2006) Ultra-high resolution optical coherence tomography assessment of photoreceptors in retinitis pigmentosa and related diseases. Am J Ophthalmol 142:945–952
Fakin A, Jarc-Vidmar M, Glavac D, Bonnet C, Petit C, Hawlina M (2012) Fundus autofluorescence and optical coherence tomography in relation to visual function in Usher syndrome type 1 and 2. Vis Res 75:60–70
Grigoropoulos VG, Emfietzoglou J, Nikolaidis P, Chatzistefanou K, Vergados J, Theodossiadis GP, Theodossiadis PG (2010) Optical coherence tomography findings in patients with retinitis pigmentosa and low visual acuity. Ophthalmic Surg Lasers Imaging 41:35–39
Ferris FL 3rd, Kassoff A, Bresnick GH, Bailey I (1982) New visual acuity charts for clinical research. Am J Ophthalmol 94:91–96
Early Treatment Diabetic Retinopathy Study design and baseline patient characteristics. ETDRS report number 7. Ophthalmology 1991;98:741–56
Bailey IL, Lovie-Kitchin JE (2013) Visual acuity testing. From the laboratory to the clinic. Vision Res 90:2–9
Holladay JT (1997) Proper method for calculating average visual acuity. J Refract Surg 13:388–391
Jolly JK, Edwards TL, Moules J, Groppe M, Downes SM, MacLaren RE (2016) A qualitative and quantitative assessment of fundus autofluorescence patterns in patients with choroideremia. Investig Ophthalmol Vis Sci 57:4498–4503
Kellner S, Kellner U, Weber BH, Fiebig B, Weinitz S, Ruether K (2009) Lipofuscin- and melanin-related fundus autofluorescence in patients with ABCA4-associated retinal dystrophies. Am J Ophthalmol 147(895–902):902.e891
Lima LH, Burke T, Greenstein VC, Chou CL, Cella W, Yannuzzi LA, Tsang SH (2012) Progressive constriction of the hyperautofluorescent ring in retinitis pigmentosa. Am J Ophthalmol 153(718–727):727.e711–727.e712
Ellabban AA, Tsujikawa A, Ooto S, Yamashiro K, Oishi A, Nakata I, Miyake M, Akagi-Kurashige Y, Ueda-Arakawa N, Arichika S, Yoshitake S, Takahashi A, Yoshimura N (2013) Focal choroidal excavation in eyes with central serous chorioretinopathy. Am J Ophthalmol 156:673–683
Robson AG, Saihan Z, Jenkins SA, Fitzke FW, Bird AC, Webster AR, Holder GE (2006) Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol 90:472–479
Bach M, Brigell M, Hawlina M, Holder G, Johnson M, McCulloch D, Meigen T, Viswanathan S (2013) ISCEV standard for clinical pattern electroretinography (PERG) – 2012 update. Doc Ophthalmol 126(126):1–7
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12
Giani A, Pellegrini M, Invernizzi A, Cigada M, Staurenghi G (2012) Aligning scan locations from consecutive spectral-domain optical coherence tomography examinations: a comparison among different strategies. Investig Ophthalmol Vis Sci 53:7637–7643
Waldstein SM, Gerendas BS, Montuoro A, Simader C, Schmidt-Erfurth U (2015) Quantitative comparison of macular segmentation performance using identical retinal regions across multiple spectral-domain optical coherence tomography instruments. Br J Ophthalmol 99:794–800
Giani A, Cigada M, Choudhry N, Deiro AP, Oldani M, Pellegrini M, Invernizzi A, Duca P, Miller JW, Staurenghi G (2010) Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol 150(815–824):e811
Aizawa S, Mitamura Y, Hagiwara A, Sugawara T, Yamamoto S (2010) Changes of fundus autofluorescence, photoreceptor inner and outer segment junction line, and visual function in patients with retinitis pigmentosa. Clin Exp Ophthalmol 38:597–604
World Health Organization. 9D90 Vision impairment including blindness: ICD-11 for mortality and morbidity statistics. 2019
Liu G, Liu X, Li H, Du Q, Wang F (2016) Optical coherence tomographic analysis of retina in retinitis pigmentosa patients. Ophthalmic Res 56:111–122
Hariri AH, Zhang HY, Ho A, Francis P, Weleber RG, Birch DG, Ferris FL 3rd, Sadda SR (2016) Trial of oral valproic acid for retinitis pigmentosa g: quantification of ellipsoid zone changes in retinitis pigmentosa using en face spectral domain-optical coherence tomography. JAMA Ophthalmol 134:628–635
Sujirakul T, Lin MK, Duong J, Wei Y, Lopez-Pintado S, Tsang SH (2015) Multimodal imaging of central retinal disease progression in a 2-year mean follow-up of retinitis pigmentosa. Am J Ophthalmol 160(786–798):e784
Battu R, Khanna A, Hegde B, Berendschot TT, Grover S, Schouten JS (2015) Correlation of structure and function of the macula in patients with retinitis pigmentosa. Eye 29:895–901
Hood DC, Lazow MA, Locke KG, Greenstein VC, Birch DG (2011) The transition zone between healthy and diseased retina in patients with retinitis pigmentosa. Investig Ophthalmol Vis Sci 52:101–108
Robson AG, El-Amir A, Bailey C, Egan CA, Fitzke FW, Webster AR, Bird AC, Holder GE (2003) Pattern ERG correlates of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 44:3544–3550
Trichonas G, Traboulsi EI, Ehlers JP (2017) Ultra-widefield fundus autofluorescence patterns in retinitis pigmentosa and other retinal dystrophies. Ophthalmic Genet 38:98–100
Robson AG, Egan CA, Luong VA, Bird AC, Holder GE, Fitzke FW (2004) Comparison of fundus autofluorescence with photopic and scotopic fine-matrix mapping in patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 45:4119–4125
Schuerch K, Woods RL, Lee W, Duncker T, Delori FC, Allikmets R, Tsang SH, Sparrow JR (2017) Quantifying fundus autofluorescence in patients with retinitis pigmentosa. Invest Ophthalmol Vis Sci 58:1843–1855
Robson AG, Tufail A, Fitzke F, Bird AC, Moore AT, Holder GE, Webster AR (2011) Serial imaging and structure-function correlates of high-density rings of fundus autofluorescence in retinitis pigmentosa. Retina 31:1670–1679
Robson AG, Lenassi E, Saihan Z, Luong VA, Fitzke FW, Holder GE, Webster AR (2012) Comparison of fundus autofluorescence with photopic and scotopic fine matrix mapping in patients with retinitis pigmentosa: 4- to 8-year follow-up. Investig Ophthalmol Vis Sci 53:6187–6195
Oishi A, Ogino K, Makiyama Y, Nakagawa S, Kurimoto M, Yoshimura N (2013) Wide-field fundus autofluorescence imaging of retinitis pigmentosa. Ophthalmology 120:1827–1834
Trichonas G, Traboulsi EI, Ehlers JP (2017) Correlation of ultra-widefield fundus autofluorescence patterns with the underlying genotype in retinal dystrophies and retinitis pigmentosa. Ophthalmic Genet 38:320–324
Lenassi E, Saihan Z, Cipriani V, Le Quesne Stabej P, Moore AT, Luxon LM, Bitner-Glindzicz M, Webster AR (2014) Natural history and retinal structure in patients with Usher syndrome type 1 owing to MYO7A mutation. Ophthalmology 121:580–587
Stingl K, Kurtenbach A, Hahn G, Kernstock C, Hipp S, Zobor D, Kohl S, Bonnet C, Mohand-Said S, Audo I, Fakin A, Hawlina M, Testa F, Simonelli F, Petit C, Sahel JA, Zrenner E (2019) Full-field electroretinography, visual acuity and visual fields in Usher syndrome: a multicentre European study. Doc Ophthalmol 02:02
Acknowledgements
The authors would like to thank Maria Korsakova for electrophysiology testing and assessment.
Funding
This study was partly funded by National Health and Medical Research Council (NHMRC) Grants APP1116360, APP1099165, APP1109056 and Ophthalmic Research Institute of Australia (ORIA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Nina Mustafic declares that she has no conflict of interest. Author Federica Ristoldo declares that she has no conflict of interest. Author Alessandro Invernizzi, declares that he has received a grant from Allergan and a Sydney Eye Hospital foundation fellowship. Author Vuong Nguyen declares that he has no conflict of interest. Author Clare Fraser declares that she has no conflict of interest. Author Robyn Jamieson declares that she is a consultant to Novartis Australia. Author John Grigg declares that he has received a speaker honorarium from Novartis Australia and is a consultant to Novartis Australia.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Sydney Eye Hospital South East Sydney Local health District Research Ethics and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mustafic, N., Ristoldo, F., Nguyen, V. et al. Biomarkers in Usher syndrome: ultra-widefield fundus autofluorescence and optical coherence tomography findings and their correlation with visual acuity and electrophysiology findings. Doc Ophthalmol 141, 205–215 (2020). https://doi.org/10.1007/s10633-020-09765-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09765-0