Abstract
Purpose
To study the potential toxic effects of intravitreal clindamycin on the retina of albino rabbits, by assessing functional and morphological retinal changes.
Methods
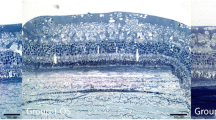
Eight albino rabbits were included in the study. In each rabbit, 1 mg/0.1 ml clindamycin was injected into the vitreous of the right (experimental) eye, and 0.1 ml saline was injected into the vitreous of the left (control) eye. The electroretinogram (ERG) was recorded before injection, 3 days, 1, 2, and 4 weeks post-injection. The visual evoked potential (VEP) was recorded 4 weeks post-injection. Clinical examination was conducted at all time points. The eyes were enucleated at the termination of the follow-up period in order to prepare the retinas for histology in order to assess retinal structure.
Results
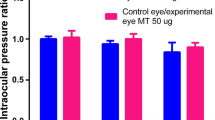
ERG and VEP responses that were recorded from the experimental eye at different times following intravitreal clindamycin injection were very similar to the corresponding responses that were recorded from the control eyes. Clinical examination was normal in all eyes, and no histological damage was observed.
Conclusions
Intravitreal injection of 1 mg clindamycin does not cause functional or morphological signs of retinal toxicity in albino rabbits, during a period of 4 weeks post-injection. These findings support the clinical use of 1 mg intravitreal clindamycin.







Similar content being viewed by others
References
Holland GN (2003) Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol 136:973–988
Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG (2009) Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 15:878–884
Harrell M, Carvounis PE (2014) Current treatment of toxoplasma retinochoroiditis: an evidence-based review. J Ophthalmol. doi:10.1155/2014/273506
Tate GW Jr, Martin RG (1977) Clindamycin in the treatment of human ocular toxoplasmosis. Can J Ophthalmol 12:188–195
Tabbara KF, O’Connor GR (1980) Treatment of ocular toxoplasmosis with clindamycin and sulfadiazine. Ophthalmology 87:129–134
Guldsten H (1983) Clindamycin and sulphonamides in the treatment of ocular toxoplasmosis. Acta Ophthalmol 61:51–57
Lakhanpal V, Schocket SS, Nirankari VS (1983) Clindamycin in the treatment of toxoplasmic retinochoroiditis. Am J Ophthalmol 95:605–613
Rothova A, Meenken C, Buitenhuis HJ et al (1993) Therapy for ocular toxoplasmosis. Am J Ophthalmol 115:517–523
Tabbara KF, Dy-Liacco J, Nozik RA, O’Connor GR, Blackman HJ (1979) Clindamycin in chronic toxoplasmosis: effect of periocular injections on recoverability of organisms from healed lesions in the rabbit eye. Arch Ophthalmol 97:542–544
Ferguson JG Jr (1981) Clindamycin therapy for toxoplasmosis. Ann Ophthalmol 13:95–100
Martinez CE, Zhang D, Conway MD, Peyman GA (1998) Successful management of ocular toxoplasmosis during pregnancy using combined intraocular clindamycin and dexamethasone with systemic sulfadiazine. Int Ophthalmol 22:85–88
Kishore K, Conway MD, Peyman GA (2001) Intravitreal clindamycin and dexamethasone for toxoplasmic retinochoroiditis. Ophthalmic Surg Lasers 32:183–192
Sobrin L, Kump LI, Foster CS (2007) Intravitreal clindamycin for toxoplasmic retinochoroiditis. Retina 27:952–957
Wong R, dell’Omo R, Marino M, Hussein B, Okhravi N, Pavesio CE (2009) Toxoplasma gondii: an atypical presentation of toxoplasma as optic disc swelling and hemispherical retinal vein occlusion treated with intravitreal clindamycin. Int Ophthalmol 29:195–198
Lasave AF, Díaz-Llopis M, Muccioli C, Belfort R Jr, Arevalo JF (2010) Intravitreal clindamycin and dexamethasone for zone 1 toxoplasmic retinochoroiditis at twenty-four months. Ophthalmology 117:1831–1838
Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, Yaseri M, Peyman GA (2011) Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology 118:134–141
Baharivand N, Mahdavifard A, Fouladi RF (2013) Intravitreal clindamycin plus dexamethasone versus classic oral therapy in toxoplasmic retinochoroiditis: a prospective randomized clinical trial. Int Ophthalmol 33:39–46
Zamora YF, Arantes T, Reis FA, Garcia CR, Saraceno JJ, Belfort R Jr, Muccioli C (2015) Local treatment of toxoplasmic retinochoroiditis with intravitreal clindamycin and dexamethasone. Arq Bras Oftalmol 78:216–219
Kim P, Younan N, Coroneo MT (2002) Hypersensitivity reaction to intravitreal clindamycin therapy. Clin Experiment Ophthalmol 30:147–148
Paque JT, Peyman GA (1974) Intravitreal clindamycin phosphate in the treatment of vitreous infection. Ophthalmic Surg 5:34–39
Stainer GA, Peyman GA, Meisels H, Fishman G (1977) Toxicity of selected antibiotics in vitreous replacement fluid. Ann Ophthalmol 9:615–618
Loewenstein A, Zemel E, Lazar M, Perlman I (1993) Drug-induced retinal toxicity in albino rabbits: the effects of imipenem and aztreonam. Invest Ophthalmol Vis Sci 34:3466–3476
McCulloch DL, Marmor M, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field electroretinography (2015 update). Doc Ophthalmol 130:1–12
Fulton AB, Hansen RM (1988) Scotopic stimulus/response relations of the b-wave of the electroretinogram. Doc Ophthalmol 68:193–304
Arden GB, Carter RM, Hogg CR, Powell DJ, Ernst WJK, Clover GM, Lyness AL, Quinlan MP (1983) A modified ERG technique and the results obtained in X-lined retinitis pigmentosa. Br J Ophthalmol 67:419–430
Peachey NS, Alexander KR, Fishman GA (1989) The luminance-response function of the dark-adapted human electroretinogram. Vision Res 29:263–270
Severns ML, Johnson MA (1993) The care and fitting of Naka-Rushton functions to electroretinographic intensity-response data. Doc Ophthalmol 85:135–150
Bresnick GH, Palta M (1987) Oscillatory potential amplitudes relation to severity of diabetic retinopathy. Arch Ophthalmol 105:929–933
Perlman I (2009) Testing retinal toxicity of drugs in animal models using electrophysiological and morphological techniques. Doc Ophthalmol 118:3–28
Perlman I (1983) Relationship between the amplitudes of the b wave and the a wave as a useful index for evaluating the electroretinogram. Br J Ophthalmol 67:443–448
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal. Prog Retina Eye Res 17:485–521
Peyman GA, Charles HC, Liu KR, Khoobehi B, Niesman M (1988) Intravitreal liposome-encapsulated drugs: a preliminary human report. Int Ophthalmol 12:175–182
Del Amo EM, Urtti A (2015) Rabbit as an animal model for intravitreal pharmacokinetics: clinical predictability and quality of the published data. Exp Eye Res 137:111–124
Acknowledgements
This study was partially supported by a grant from the Claire and Amedee Maratier Institute for the Study of Blindness and Visual Disorders, Sackler Faculty of Medicine, Tel Aviv University, Israel.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Anat Loewenstein is consultant to Allergan, Inc.; Alcon, Ltd.; Bayer healthcare, Inc.; Notal Vision, Ltd.; Novartis, Inc.; Teva, Ltd. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in this study were in accordance with the ethical standards of the institution at which the study was conducted.
Statement of human rights
This article does not contain any studies with human participants performed by any of the authors.
Statement on the welfare of animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
As this article does not contain any studies with human participants performed directly by any of the authors, the concept of informed consent is not applicable.
Additional information
Zohar Habot-Wilner and Orit Mazza have contributed equally as first authors.
Rights and permissions
About this article
Cite this article
Habot-Wilner, Z., Mazza, O., Shahar, J. et al. Safety of intravitreal clindamycin in albino rabbit eyes. Doc Ophthalmol 135, 133–146 (2017). https://doi.org/10.1007/s10633-017-9599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-017-9599-5