Abstract
Purpose
We investigated how the N-methyl-dl-aspartic acid (NMDA) receptor contributes to generating oscillatory potentials (OPs) of the electroretinogram (ERG) in the Royal College of Surgeons (RCS) rat.
Methods
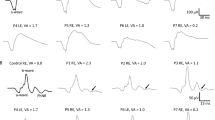
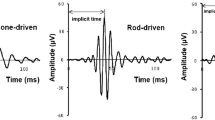
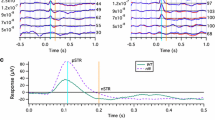
Scotopic ERGs were recorded from dystrophic and wild-type congenic (WT) RCS rats (n = 20 of each) at 25, 30, 35, and 40 days of age. The stimulus intensity was increased from −2.82 to 0.71 log cd-s/m2 to obtain intensity-response function. NMDA was injected into the vitreous cavity of the right eyes. The left eyes were injected with saline as controls. The P3 obtained by a-wave fitting was digitally subtracted from the scotopic ERG to isolate the P2. For the OPs, the P2 was digitally filtered between 65 and 500 Hz. The amplitudes of OP1, OP2, OP3, and OP4 were then measured and summed and designated as ΣOPs. The implicit times of OP1, OP2, and OP3 were also measured. The frequency spectra of the OPs were analyzed using fast Fourier transform (FFT).
Results
The maximum ERG a- and b-waves as well as ΣOPs amplitudes reduced with age in dystrophic rats. Compared with intravitreal saline injection, administration of NMDA decreased ΣOPs amplitudes from 30 days of age in dystrophic rats, while it did not attenuate ΣOPs amplitudes in WT rats. The implicit times of the OPs of the maximum ERG were prolonged by NMDA injections in WT and dystrophic rats. NMDA/saline ratios of ΣOPs amplitudes area under the FFT curves were significantly lower in dystrophic rats from 30 days of age than that in WT rats.
Conclusion
In the early stage of photoreceptor degeneration, intravitreal NMDA injection attenuated OPs amplitudes in dystrophic rats. This indicates that NMDA receptors play a significant role in generating OPs amplitudes with advancing photoreceptor degeneration.







Similar content being viewed by others
References
Dowling JE, Sidman RL (1962) Inherited retinal dystrophy in the rat. J Cell Biol 14:73–109
D’Cruz PM, Yasumura D, Weir J, Matthes MT, Abderrahim H, LaVail MM, Vollrath D (2000) Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human Mol Genet 9:645–651
Duncan JL, LaVail MM, Yasumura D, Matthes MT, Yang H, Trautmann N, Chappelow AV, Feng W, Earp HS, Matsushima GK, Vollrath D (2003) An RCS-like retinal dystrophy phenotype in mer knockout mice. Invest Ophthalmol Vis Sci 44:826–838
Feng W, Yasumura D, Matthes MT, LaVail MM, Vollrath D (2002) Mertk triggers uptake of photoreceptor outer segments during phagocytosis by cultured retinal pigment epithelial cells. J Biol Chem 277:17016–17022
Vollrath D, Feng W, Duncan JL, Yasumura D, D’Cruz PM, Chappelow A, Matthes MT, Kay MA, LaVail MM (2001) Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci USA 98:12584–12589
Gal A, Li Y, Thompson DA, Weir J, Orth U, Jacobson SG, Apfelstedt-Sylla E, Vollrath D (2000) Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nature Genet 26:270–271
McHenry CL, Liu Y, Feng W, Nair AR, Feathers KL, Ding X, Gal A, Vollrath D, Sieving PA, Thompson DA (2004) MERTK arginine-844-cysteine in a patient with severe rod-cone dystrophy: loss of mutant protein function in transfected cells. Invest Ophthalmol Vis Sci 45:1456–1463
LaVail MM (2001) Legacy of the RCS rat: impact of a seminal study on retinal cell biology and retinal degenerative diseases. Prog Brain Res 131:617–627
Chader GJ (2002) Animal models in research on retinal degenerations: past progress and future hope. Vis Res 42:393–399
Sieving PA, Frishman LJ, Steinberg RH (1986) Scotopic threshold response of proximal retina in cat. J Neurophysiol 56:1049–1061
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136
Bush RA, Hawks KW, Sieving PA (1995) Preservation of inner retinal responses in the aged Royal College of Surgeons rat. Evidence against glutamate excitotoxicity in photoreceptor degeneration. Invest Ophthalmol Vis Sci 36:2054–2062
Ohzeki T, Machida S, Takahashi T, Ohtaka K, Kurosaka D (2007) The effect of intravitreal N-methyl-DL- aspartic acid on the electroretinogram in Royal College of Surgeons rats. Jpn J Ophthalmol 51:165–174
Machida S, Raz-Prag D, Fariss RN, Sieving PA, Bush RA (2008) Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci 49:442–452
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal. Prog Retin Eye Res 17:485–521
Yonemura D, Aoki T, Tsuzuki K (1962) Electroretinogram in diabetic retinopathy. Arch Ophthalmol 68:19–24
Shirao Y, Kawasaki K (1998) Electrical responses from diabetic retina. Prog Retin Eye Res 17:59–76
Banin E, Cideciyan AV, Aleman TS, Petters RM, Wong F, Milam AH, Jacobson SG (1999) Retinal rod photoreceptor-specific gene mutation perturbs cone pathway development. Neuron 23:549–557
Sakai T, Kondo M, Ueno S, Koyasu T, Komeima K, Terasaki H (2009) Supernormal ERG oscillatory potentials in transgenic rabbit with rhodopsin P347L mutation and retinal degeneration. Invest Ophthalmol Vis Sci 50:4402–4409
Ikenoya K, Kondo M, Piao CH, Kachi S, Miyake Y, Terasaki H (2007) Preservation of macular oscillatory potentials in eyes of patients with retinitis pigmentosa and normal visual acuity. Invest Ophthalmol Vis Sci 48:3312–3317
Slaughter MM, Miller RF (1983) The role of excitatory amino acid transmitters in the mudpuppy retina: an analysis with kainic acid and N-methyl aspartate. J Neurosci 3:1701–1711
Akula JD, Mocko JA, Moskowitz A, Hansen RM, Fulton AB (2007) The oscillatory potentials of the dark-adapted electroretinogram in retinopathy of prematurity. Invest Ophthalmol Vis Sci 48:5788–5797
Hood DC, Birch DG (1996-1997) Assessing abnormal rod photoreceptor activity with the a-wave of the electroretinogram: applications and methods. Doc Ophthalmol. 92: 253-267
Lamb TD, Pugh EN Jr (1992) A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol 449:719–758
Zhang K, Yao G, Gao Y, Hofeldt KJ, Lei B (2007) Frequency spectrum and amplitude analysis of dark- and light-adapted oscillatory potentials in albino mouse, rat and rabbit. Doc Ophthalmol 115:85–93
Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D (2006) Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. Jpn J Ophthalmol 50:367–373
Takahashi T, Machida S, Masuda T, Mukaida Y, Tazawa Y (2005) Functional changes in rod and cone pathways after photoreceptor loss in light-damaged rats. Curr Eye Res 30:703–713
Koyasu T, Kondo M, Miyata K, Ueno S, Miyata T, Nishizawa Y, Terasaki H (2008) Photopic electoretinograms of mGluR6-deficient mice. Curr Eye Res 33:91–99
Hughes A (1979) A schematic eye for the rat. Vis Res 19:569–588
Vaegan, Millar TJ (1994) Effect of kainic acid and NMDA on the pattern electroretinogram, the scotopic threshold response, the oscillatory potentials and the electroretinogram in the urethane anaesthetized cat. Vis Res 34:1111–1125
Narahashi T, Moore JW, Scott WR (1964) Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J Gen Physiol 47:965–974
Narahashi T (1974) Chemicals as tools in the study of excitable membranes. Physiol Rev 54:813–889
Bloomfield SA (1996) Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol 75:1878–1893
Tanaka M, Machida S, Ohtaka K, Tazawa Y, Nitta J (2005) Third-order neuronal responses contribute to shaping the negative electroretinogram in sodium iodate-treated rats. Curr Eye Res 30:443–453
Jansen HG, Sanyal S (1984) Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol 224:71–84
Jansen HG, Sanyal S (1987) Synaptic changes in the terminals of rod photoreceptors of albino mice after partial visual cell loss induced by brief exposure to constant light. Cell Tissue Res 250:43–52
Jansen HG, Sanyal S (1992) Synaptic plasticity in the rod terminals after partial photoreceptor cell loss in the heterozygous rds mutant mouse. J Comp Neurol 316:117–125
Jones BW, Watt CB, Frederick JM, Baehr W, Chen CK, Levine EM, Milam AH, Lavail MM, Marc RE (2003) Retinal remodeling triggered by photoreceptor degenerations. J Comp Neurol 464:1–16
Marc RE, Jones BW (2003) Retinal remodeling in inherited photoreceptor degenerations. Mol Neurobiol 28:139–147
Grunder T, Kohler K, Guenther E (2001) Alterations in NMDA receptor expression during retinal degeneration in the RCS rat. Vis Neurosci 18:781–787
Malenka RC, Nicoll RA (1993) NMDA-receptor-dependent synaptic plasticity: multiple forms and mechanisms. Trends Neurosci 16:521–527
Collingridge GL, Bliss TV (1995) Memories of NMDA receptors and LTP. Trends Neurosci 18:54–56
Constantine-Paton M (2000) The plastic brain. Neurobiol Dis 7:515–519
Pothecary CA, Thompson H, Salt TE (2005) Changes in glutamate receptor function in synaptic input to the superficial superior colliculus (SSC) with aging and in retinal degeneration in the Royal College of Surgeons (RCS) rat. Neurobiol Aging 26:965–972
Acknowledgments
This study was supported by a Grant (#17591850) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (SM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harada, T., Machida, S., Nishimura, T. et al. Contribution of N-methyl-dl-aspartic acid (NMDA)-sensitive neurons to generating oscillatory potentials in Royal College of Surgeons rats. Doc Ophthalmol 127, 131–140 (2013). https://doi.org/10.1007/s10633-013-9394-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-013-9394-x