Abstract
Background
Treatment with atezolizumab and bevacizumab has become standard of care for advanced unresectable hepatocellular carcinoma (HCC) but carries an increased gastrointestinal bleeding risk. Therefore, patients are often required to undergo esophagogastroduodenoscopy (EGD) to rule out esophageal varices (EV) prior to initiating therapy, which can delay care and lead to unnecessary procedural risks and health care costs. In 2019, the EVendo score was created and validated as a noninvasive tool to accurately screen out patients who were at low risk for having EV that required treatment. We sought to validate whether the EVendo score could be used to accurately predict the presence of EV and varices needing treatment (VNT) in patients with HCC.
Methods
This was a retrospective multicenter cohort study of patients with HCC from 9/2004 to 12/2021. We included patients who underwent EGDs within 1 year after their HCC diagnosis. We collected clinical parameters needed to calculate an EVendo score at the time of EGD and compared the EVendo model prediction to the gold standard endoscopic report in predicting presence of VNT.
Results
112 with HCC were recruited to this study, with 117 qualifying EGDs. VNT occurred in 39 (33.3%) patients. The EVendo score had a sensitivity of 97.4% and a negative predictive value of 96.9%, supporting the validity in applying EVendo in predicting VNT in HCC.
Conclusion
In this study, we validated the use of the EVendo score in ruling out VNT in patients with HCC. The application of the EVendo score could safely defer about 30% of EGDs for EV screening in HCC patients. Although additional validation cohorts are needed, this suggests that EVendo score can potentially be applied in patients with HCC to avoid unnecessary EGDs, which can ultimately mitigate healthcare costs and delays in initiating HCC treatment with atezolizumab and bevacizumab.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For advanced unresectable hepatocellular carcinoma (HCC), treatment with atezolizumab and bevacizumab has become the standard first-line therapy since the IMBrave 150 trial in 2020 [1]. However, treatment with antiangiogenic agents is known to carry increased gastrointestinal bleeding risk [2, 3]. Specifically in the HCC population, the risk of bleeding is a significant concern given patients often have underlying cirrhosis, portal hypertension, and thrombocytopenia. Therefore, in the IMBrave 150 trial, patients were required to have an esophagogastroduodenoscopy (EGD) prior to enrollment to detect esophageal varices (EV) [1]. Although there are currently no international standardized criteria for mandated endoscopy prior to treatment in advanced HCC, HCC patients commonly undergo EGD to evaluate for presence of varices before initiation of treatment [4, 5]. However, this is associated with procedural risks, and can lead to delays in care and increased healthcare costs.
Recently, a real-world multicenter analysis compared HCC patients treated with atezolizumab and bevacizumab who received EGD prior to treatment versus no EGD [6]. They found no difference in bleeding events between the two groups, presumably due to physician judgement of low risk of portal hypertension in the group that did not receive endoscopy. This supports a selective approach to EGD, in which patients with low risk of bleeding may safely forego endoscopy. This highlights the need for non-invasive tools that can serve as primary screening methods to limit unnecessary EGD in patients with HCC.
In the literature, there are several noninvasive predictive factors identified for EV bleeding. Well studied predictive factors for EV bleeding events include a Child–Pugh Class grade B or above, a fibrosis index score greater than 3.95, a FIB-4 score greater than 3.3, or a high liver stiffness and spleen stiffness score [7,8,9,10]. Most of these factors evaluated for an EV bleed event, but were not studied for the presence of VNT. While all these factors have been evaluated in cirrhotic patients, they have not been validated specifically in the HCC population. Other associated factors with EV bleeding include presence of ascites, low fibrinogen, low albumin, elevated creatinine, and elevated bilirubin levels, but these have not been studied enough as robust predictive factors on their own [7].
The Baveno VI criteria, which relies on liver stiffness measurement (LSM) and platelet counts, is a current screening method for EV that was recently validated in HCC patients. The Baveno VI criteria had a < 5% VNT miss rate and spared about 20% of patients from receiving an EGD [2]. Nevertheless, this criterion requires the availability of LSM within a year, which may not be available in all clinical settings [2]. Therefore, there is a need for additional noninvasive VNT screening methods specific for HCC patients. In 2019, the EVendo was developed and validated as a noninvasive model to predict the presence of esophageal varices (EV) in non-HCC cirrhotic patients [11, 12]. The score considers international normalized ratio (INR), aspartate aminotransferase (AST), platelet count, urea nitrogen, hemoglobin, and presence of ascites. An EVendo score less than 3.90 suggesting low risk of varices needing treatment (VNT). While the EVendo score is validated in liver cirrhosis, its use in advanced HCC patients has not been studied. Our study aims to investigate whether the EVendo score could be used to accurately predict the presence of EV and VNT in patients with HCC.
Methods
Study Design and Participants
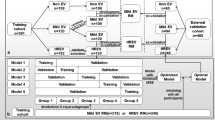
This was a retrospective multicenter cohort study conducted at University of California, Los Angeles and the Veteran Affairs Hospital in West Los Angeles. The patient cohort included a retrospective review of all adults diagnosed with HCC between 9/2004 and 12/2021. HCC was diagnosed by ICD9/ICD10 codes, liver biopsy showing HCC, or by MRI showing LI-RADS 5 lesion. The inclusion criteria included patients diagnosed with HCC at any stage who received an EGD less than 1 year following their HCC diagnosis. Patients were excluded from the study if they never received an EGD after HCC diagnosis, received an EGD greater than 1 year after HCC diagnosis or before HCC diagnosis, or did not have lab information available at the time of EGD. All inclusion and exclusion criteria were determined by two physician manual chart review of the electronic health record. The variables collected included demographic data such as age, gender, race. We collected prognostic scores such as MELD-Na score, Childs-Turcotte-Pugh (CTP) score calculated from lab information at the time of the EGD. Additionally, we looked at whether the patient had cirrhosis, and if so, the etiology of cirrhosis, the initial treatment for HCC, and whether tumors were within or outside of Milan criteria. Additionally, clinical parameters needed to calculate the EVendo score were collected at the time of the EGD including international normalized ratio (INR), aspartate aminotransferase (AST), platelet count, blood urea nitrogen (BUN), hemoglobin, and presence of ascites, as determined by imaging or hepatology consult notes.
We compared the EVendo model prediction to the gold standard endoscopic report in predicting presence of EV. VNT was defined as medium or large varices, or small varices with high-risk stigmata. Varices not needing treatment (VNNT) were defined as small varices without high-risk stigmata or no EV [13, 14].
Statistical Analysis
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) was calculated to evaluate accuracy of EVendo score. Missed VNT percentage was calculated as false negative cases divided by total number of patients.
Results
The cohort contained 112 patients and 117 EGDs. A total of 33.3% had VNT and 66.6% had VNNT or no varices. The study population was 80.3% male and 19.7% female. The average age was 65.8 years and 53% were non-white. For the VNT population, there were 41% that were CTP A, 38% that were CTP B, and 15.4% that were CTP C. In comparison, for the VNNT population, 66.6% that were CTP A, 17.9% that were CTP B, and 10.2% that were CTP C (p = 0.019). There was a significant difference in CTP score between the VNT and VNNT group, mainly driven by the larger percentage of CTP A patients in the VNNT group. Between the VNT and VNNT group, there were no significant differences in the MELD-Na score (VNT 12.9 ± 0.9), NNT 11.6 ± 5.3, p = 0.12), distribution of age (VNT 63.3 ± 7.03, VNNT 64.6 ± 7.09, p = 0.07), distribution of gender (VNT 76.9% male, VNNT 82.1% male, p = 0.31), or percentage of patients with cirrhosis (VNT 94.8%, VNNT 92.3%, p = 0.61). There was no significant difference in etiology of cirrhosis or liver disease between the two groups: Hepatitis C (VNT 58%, VNNT 53.8%), Hepatitis B (VNT 12.8%, VNNT 2.5%), metabolic-associated steatohepatitis (VNT 10.3%, VNNT 3.8%), alcohol (VNT 10.3%, VNNT 11.5%), cryptogenic (VNT 2.5%, VNNT 0%), PSC/PBC (VNT 5.1%, VNNT 1.3%), multifactorial (VNT 10.3%, VNNT 20.5%) (p = 0.08). There was no significant difference in Barcelona-Clinic Liver Cancer (BCLC) Staging between the two groups: BCLC A (VNT 56.4%, VNNT 57.7%), BCLC B (VNT 17.9%, VNNT 19.2%), BCLC C (VNT 12.8%, VNNT 17.9%), BCLC D (VNT 10.3%, VNNT 1.3%). There was no significant difference in initial treatment for HCC: resection (VNT 0%, VNNT 3.8%), radiofrequency ablation (VNT 30.8%, VNNT 33.3%), trans-arterial chemoembolization (VNT 25.6%, VNNT 15.4%), Y90 Treatment (VNT 7.7%, VNNT 2.6%), Atezolizumab/bevacizumab or sorafenib (VNT 10.3%, VNNT 19.2%), transplant (VNT 5.1%, VNNT 3.8%), other treatment (VNT 5.1%, VNNT 12.8%), and unknown (VNT 2.6%, VNNT 12.8%). (p = 0.201). In summary, the most common initial treatments in both groups were trans-arterial chemoembolization and radiofrequency ablations. This was followed by atezolizumab and bevacizumab treatment, and less commonly, hepatic resection, Y9 treatment, transplant, and other or unknown treatments. Overall, 66 patients had tumors within Milan criteria, and 49 had tumors outside Milan criteria. The baseline characteristics of all the patients are shown in Table 1.
Of the 117 EGDs performed, the EVendo score had a sensitivity of 97.4%, specificity of 41.0% for VNT, positive predictive value of 45.2%, and a negative predictive value of 96.9%. A total of 32 EGDs, or 27.3% of EGDs in this cohort, could have been deferred if the EVendo score was applied. The missed VNT percentage for the EVendo score was 2.56% (1/39). The confusion matrix depicting these results are shown in Table 2.
We also performed a sub-analysis of the EVendo score performance in patients who were treated with atezolizumab and bevacizumab for HCC (Table 3). Of the 19 EGDs that were performed, the EVendo score had a sensitivity of 100%, specificity of 46.7%, positive predictive value of 30%, and a negative predictive value of 100% (Table 3). 36.8% of EGDs in this cohort could have been deferred if the EVendo score was applied.
We also performed an additional analysis assessing EVendo score performance in detecting VNT across the different HCC stages through assessing BCLC staging and Milan criteria. The EVendo score had a negative predictive value of 95.5% in BCLC Stage A, and 100% in BCLC Stages B-D. The missed VNT percentage was < 5% in all BCLC stages (Supplemental Tables 1 and 2). Looking into Milan criteria, the negative predictive value was 95.2% in patients who met Milan criteria, and 100% in patients who did not meet Milan criteria (Supplemental Tables 3 and 4). This suggests that the EVendo score can be applied across all stages of HCC.
Discussion
Many patients who undergo screening EGDs are found to have no varices or varices that do not need treatment. In this study, about 66.6% of patients who underwent EGD screening for EV did not have endoscopic evidence of VNT. Particularly in patients with HCC, time to initial treatment is important to prevent cancer progression and, which can be delayed by unnecessary EGDs. Given that treatment with atezolizumab and bevacizumab increases the risk of bleeding, there is a need for high-performing non-invasive screening tests for risk stratification in HCC patients.
The performance of the EVendo score in HCC patients in this study is promising, with a cutoff score of < 3.90 giving high negative predictive values in ruling out VNT and very low missed VNT percentages. In this validation cohort of patients with HCC, the EVendo score had a NPV of 96.9% and a missed VNT percentage of 2.56%. These performance statistics were similar to the original EVendo study by Dong et al. evaluating EVendo performance in predicting VNT in patients with cirrhosis [11]. The NPV in the original cirrhotic training cohort was 95.8% [11]. The validation cohort of 109 cirrhotic patients undergoing EV screening prospectively had an NPV of 100% [11]. A validation of the EVendo score for the prediction of varices for patients with cirrhosis in a separate cohort was performed by Alswat et al. Using the same cutoff score of EVendo < 3.90, they found the NPV for VNT was reliable at 94% [12]. The Baveno VI criteria is another noninvasive risk stratification tool that has been well validated to predict varices in patients with liver cirrhosis [15,16,17]. In the Baveno VI criteria, a liver stiffness measurement (LSM) less than or equal to 20 kPa by transient elastography or platelet count greater than or equal to 150 × 109 predicts a low risk of VNT. The Baveno VII criteria extends its application to all patients with cirrhosis and clinically significant portal hypertension using liver stiffness measurement less than or equal to 15 kPa and a platelet count greater than or equal to 150 × 109 [2, 18]. Recently in May 2023, the Baveno VII criteria was also validated for use in patients with hepatocellular carcinoma with a VNT missed rate of < 5% [2]. This study shows that the EVendo model has a similar VNT missed rate of < 5% for HCC patients. Because the Baveno criteria requires an LSM within a year, the EVendo score is an attractive alternative as it utilizes commonly collected labs.
A strength of our study is that it is the first validation study for the EVendo score for HCC patients in an external cohort. Additionally, the patients that were included were from real world clinical practice with a multicenter design allowing for a diverse and more generalizable population. Several limitations need to be considered in interpreting our findings. First, the study was a retrospective analysis which carries the potential for bias including selection or misclassification bias. However, we attempted to mitigate this by applying strict inclusion and exclusion criteria. Reassuringly, majority of validation studies for non-invasive screening tests for varices have been retrospective [12, 19], but have shown similar results to prospective studies [20, 21]. Second, noninvasive prediction of VNT are more important for those with compensated cirrhosis or CTP A than those with decompensated cirrhosis or CTP grade B or higher. Although we looked at 117 patients, only 68 patients were CTP A. More validation studies in CTP A patients would be beneficial in the future. Finally, in any varices screening study, there is inevitable intra-operator variation among endoscopists in interpreting presence of VNT and VNNT.
Conclusion
The EVendo score was 97.4% sensitive and had a 96.9% negative predictive value in predicting VNT in a cohort of patients with HCC. In patients who received atezolizumab and bevacizumab, the EVendo score showed similar results with a sensitivity of 100%, and a negative predictive value of 100%. These results are comparable to application of the Baveno VII criteria for this population and may be useful particularly in resource-limited settings. Although additional validation cohorts are needed, this suggests that EVendo score can potentially be applied in patients with HCC to avoid unnecessary EGDs, which can ultimately mitigate healthcare costs and delays in initiating HCC treatment with atezolizumab and bevacizumab.
Data availability
The data that support the findings of this study are available on request from the corresponding author, TD. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Abbreviations
- AST:
-
Aspartate aminotransferase
- BUN:
-
Blood urea nitrogen
- CTP:
-
Child Turcotte Pugh
- EGD:
-
Esophagogastroduodenoscopy
- EV:
-
Esophageal varices
- HCC:
-
Hepatocellular carcinoma
- INR:
-
International normalized ratio
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- VNT:
-
Varices needing treatment
- VNNT:
-
Varices not needing treatment
References
Finn RS, Qin S, Ikeda M et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905.
Wu CW, Lui RN, Wong VW et al. Baveno VII criteria is an accurate risk stratification tool to predict high-risk varices requiring intervention and hepatic events in patients with advanced hepatocellular carcinoma. Cancers. 2023;15:2480.
Iavarone M, Primignani M, Vavassori S et al. Determinants of esophageal varices bleeding in patients with advanced hepatocellular carcinoma treated with sorafenib. United Eur Gastroenterol J. 2016;4:363–370.
Gordan JD, Kennedy EB, Abou-Alfa GK et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol. 2020;38:4317–4345.
Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol. 2021;75:960–974.
LEE CL, Freeman M, O'Donnell C et al. Real-world multicentre analysis of patients with hepatocellular carcinoma (HCC) treated with atezolizumab and bevacizumab (A+B): Efficacy, esophagogastroduodenoscopy (EGD) uptake and bleeding complications. J Clin Oncol. 2023;41:4105–4105.
Guinazu C, Fernandez Munoz A, Maldonado MD et al. Assessing the predictive factors for bleeding in esophageal variceal disease: A systematic review. Cureus. 2023;15:e48954.
Kraja B, Mone I, Akshija I, Kocollari A, Prifti S, Burazeri G. Predictors of esophageal varices and first variceal bleeding in liver cirrhosis patients. World J Gastroenterol. 2017;23:4806–4814.
Wang SF, Huang YT, Huang CH, Chang SH, Lin CY. Fibrosis index predicts variceal bleeding and reduces need for nonselective beta-blocker in compensated cirrhosis with initial small esophageal varices without red-color sign. Ann Transl Med. 2020;8:1223.
Liu Y, Tan HY, Zhang XG, Zhen YH, Gao F, Lu XF. Prediction of high-risk esophageal varices in patients with chronic liver disease with point and 2D shear wave elastography: a systematic review and meta-analysis. Eur Radiol. 2022;32:4616–4627.
Dong TS, Kalani A, Aby ES et al. Machine learning-based development and validation of a scoring system for screening high-risk esophageal varices. Clin Gastroenterol Hepatol. 2019;17:1894–1901.e1.
Alswat K, Alanazi M, Bashmail A et al. Validation of the EVendo score for the prediction of varices in cirrhotic patients. Saudi J Gastroenterol. 2022;28:378–384.
North Italian Endoscopic Club for the S, Treatment of Esophageal V. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. A prospective multicenter study. N Engl J Med. 1988;319:983–989.
Reiberger T, Puspok A, Schoder M et al. Austrian consensus guidelines on the management and treatment of portal hypertension (Billroth III). Wien Klin Wochenschr. 2017;129:135–158.
Pizzamiglio M, Weicker A, de Terwangne C, Henrion J, Descamps OS, De Vos M. Validation of Baveno VI and expanded-Baveno VI criteria for predicting gastroesophageal varices in patients with alcoholic and non-alcoholic fatty liver disease. Acta Gastroenterol Belg. 2022;85:321–329.
Wang H, Wen B, Chang X et al. Baveno VI criteria and spleen stiffness measurement rule out high-risk varices in virally suppressed HBV-related cirrhosis. J Hepatol. 2021;74:584–592.
Petta S, Sebastiani G, Bugianesi E et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69:878–885.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII—Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974.
Bae J, Sinn DH, Kang W et al. Validation of the Baveno VI and the expanded Baveno VI criteria to identify patients who could avoid screening endoscopy. Liver Int. 2018;38:1442–1448.
Nawalerspanya S, Sripongpun P, Chamroonkul N, Kongkamol C, Piratvisuth T. Validation of original, expanded Baveno VI, and stepwise & platelet-MELD criteria to rule out varices needing treatment in compensated cirrhosis from various etiologies. Ann Hepatol. 2020;19:209–213.
Giannini EG, Zaman A, Kreil A, et al. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511–2519.
Author information
Authors and Affiliations
Contributions
JY, TD wrote the main manuscript text and prepared tables. TD was responsible for conceptualization and supervision of the project. PC, JB, JY, AP aided with methodology and data collection. All authors reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no disclosures, financial, professional, or personal to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yang, J.O., Chittajallu, P., Benhammou, J.N. et al. Validation of a Machine Learning Algorithm, EVendo, for Predicting Esophageal Varices in Hepatocellular Carcinoma. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08449-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08449-y