Abstract
Background
Functional Abdominal Bloating and Distension (FABD) is a multifaceted condition related in part to trapped gas, with changes in the intestinal barrier and small intestinal bacterial overgrowth (SIBO), which lead to gas production. Currently, there are no treatments targeting the etiology of FABD.
Methods
This double-blind, multicenter, randomized study evaluated the safety and efficacy of a product containing xyloglucan and pea proteins (XG + PP) compared with simethicone, both administered orally (three times daily) for 20 consecutive days. Eighty-eight patients with FABD were randomly assigned to the two groups in a 1:1 ratio. Primary outcome was safety; secondary outcomes were (i) efficacy in alleviating the symptoms of FABD and (ii) efficacy in reducing SIBO, as assessed by hydrogen breath test (HBT).
Results
No Adverse Events or Serious Unexpected Adverse Reactions were reported during the study. XG + PP showed a faster onset of action and a significant reduction in bloating and abdominal pain compared with simethicone. At Day 20, XG + PP drastically reduced abdominal girth when compared with simethicone, with an average reduction of 4.7 cm versus 1.8 cm. At Day 20, the XG + PP arm showed a significant reduction in HBT compared to baseline.
Conclusions
This study supports the evidence that FABD patients may benefit from a XG + PP-based treatment that acts on etiology and not just the symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Functional abdominal bloating and distension (FABD) is a common gastrointestinal disorder affecting 3.5–31% of the general population, and up to 75% of patients with constipation-associated irritable bowel syndrome [1,2,3]. As defined by the Rome IV criteria, FABD is characterized by recurrent symptoms such as abdominal fullness, a feeling of pressure, the sensation of trapped gas, and/or a measurable increase in abdominal girth [4, 5]. FABD symptoms vary in severity throughout the day—worsening during the day and stabilizing at night—and they can be exacerbated by poor lifestyle, such as eating heavy meals [6]. Bloating is an uncomfortable sensation that severely affects quality of life (QoL) and limits physical functions and daily activities such as eating, mobility in bed, and toileting [7].
Several possible factors contributing to the occurrence of bloating have been identified, including visceral hypersensitivity [8, 9], behavioral abnormal abdominal wall-phrenic reflexes and perception [10, 11], abnormal gastrointestinal motility [8, 12], pelvic floor dysfunction [13], and microbiome alterations [14, 15]. There is growing evidence that carbohydrate and polyol intolerance, intestinal dysbiosis, and bacterial and methanogen overgrowth of the small intestine are involved in the pathophysiology of FABD [16, 17]. Indeed, bacterial overgrowth can trigger an inflammatory response in the intestinal mucosa and exacerbate the symptoms of FABD, contributing to intestinal barrier disruption [4, 18, 19].
Current treatment options for FABD include dietary changes, antibiotics, probiotics, and prebiotics; stimulants for motility and secretion of intestinal fluid; neuromodulators; prokinetic agents; biofeedback; hypnotherapy [9, 20,21,22]; antispasmodics [9]; and natural products (such as peppermint oil or Rikkunshito, a Japanese herbal medicine) [23,24,25].
Simethicone is an inert substance with antifoaming properties that alters the surface tension of gas bubbles [26], thus reducing the occurrence and intensity of flatulence and bloating [27]. While simethicone is commonly used to treat FABD to reduce gastrointestinal bloating, it only acts on the symptoms, and not the cause of the disease.
An ideal therapeutic approach could be based on treating the etiology of FABD to prevent the onset of symptoms. Recently, an innovative product containing a combination of xyloglucan (XG) and pea protein (PP) has been developed to act on the etiology of the disease by protecting the intestinal walls and preventing the adhesion and proliferation of pathogens. XG is a hemicellulose found in the primary cell walls of all vascular plants and is part of the human diet. Thanks to its ‘mucin-like’ molecular structure, XG forms a protective film on the intestinal epithelium that can increase the resistance of the mucosa to intestinal pathogens and allow the restoration of normal intestinal function [28]. For this reason, XG has been used primarily to control and minimize gastrointestinal symptoms of various etiologies, such as abdominal pain and frequent defecation [29]. It has been shown that the efficacy of XG increases when it is co-administered with pea proteins (PP). In fact, PP and XG have been shown to act synergistically reducing intestinal hyperpermeability by restoring tight junction expression [18, 30].
Therefore, the aim of this multicenter, randomized, double-blind study was to compare the safety and efficacy of XG + PP versus simethicone, in a cohort of FABD patients, to investigate the possibility of an alternative treatment option that can address the causes—not just the symptoms—of the disease.
Materials and Methods
Study Design
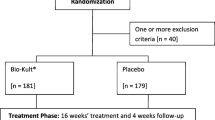
This multicentre, double-blind, randomized, parallel-group study was conducted in four gastroenterology medical centers, three in Bulgaria and one in Romania. The study was conducted between December 2019 and June 2020.
The study was approved by Ethics Committee and conducted in accordance with the revised Declaration of Helsinki. It was registered in the ISRCTN (ISRCTN70821789). Subjects were allowed to withdraw from the study at any time without giving a reason. Investigators could exclude participants for ethical reasons or if the treatment was considered harmful to the patients’ welfare.
Patients were enrolled by gastroenterology specialists or primary care physicians. Inclusion criteria were male or female subjects aged 18 to 65 years, with a diagnosis of functional abdominal bloating and distension according to the Rome IV criteria. All subjects were required to provide written informed consent to participate in the study prior to screening. Exclusion criteria included: pregnancy or breastfeeding; unwillingness to sign the informed consent form; allergy or hypersensitivity to any of the product ingredients; inability to participate to the study visits; health condition that in the opinion of the investigator, precluded participation in the study; diabetes, celiac disease; antibiotic treatment two weeks before the hydrogen breath test (HBT); and use of laxatives within two weeks before the HBT.
Using a computer-generated randomization scheme, subjects were selected in a 1:1 ratio to receive XG + PP or simethicone (Arkogas containing 257.5 mg simethicone, Arkopharma Srl) for 20 consecutive days. Treatments were administered orally three times daily according to the instructions of the product leaflet. To ensure double blinding, the capsules of XG + PP and simethicone were identical, and specific codes for the products representing the two groups were distributed to the centers. Allocation concealment was ensured by a third-party company. For this purpose, the retro-blister of both products was completely covered with a label and the two products were packed in neutral, identical, and labeled boxes. The label of each box was identical except for the randomization number. Each randomization number, along with the respective product code, was entered into the randomization list, which remained blinded until the end of the study.
Treatment adherence was monitored by counting the number of tablets. Patient demographic and medical data were collected at the beginning of the study. Subjects were examined four times: at baseline (Visit 1); after 2 days of treatment (Visit 2); after 10 days of treatment (Visit 3); and after 20 days of treatment (Visit 4, end of treatment). Subjects underwent a hydrogen breath test at Visit 1 and Visit 4. The following examinations were performed at baseline and at each study visit: abdominal circumference measurement, general medical evaluation; concomitant medication recording and safety assessment.
Study Objectives and Study Endpoints
The primary objective of the study was to evaluate the safety of XG + PP in adult patients with FABD. Safety was assessed by the occurrence of adverse effects during the study (frequency, severity, and association with study treatments) and by clinical criteria and vital signs collected at each study visit.
As a secondary outcome, the clinical efficacy of XG + PP vs. simethicone was assessed by (i) patient self-assessment of symptoms on the Visual Analogue Scale (VAS) for bloating, abdominal pain, distension, flatulence, (ii) abdominal circumference, and (iii) SIBO.
The VAS scale ranged from “no complaints” (score 0) to “severe complaints” (score 10) and symptom severity was assessed before, 60, and 120 min after each administration of XG + PP or simethicone for 8 days. Measurements were usually taken in the morning before breakfast. Abdominal circumference was measured in centimeters at the end of a normal exhalation at each study visit using a circumference meter. To compare the clinical efficacy of XG + PP with that of simethicone in reducing SIBO, subjects performed the HBT at baseline and Day 20. The HBT was performed under fasting conditions before and after taking 50 g of glucose in 250 mL of water, following all recommendations of the European consensus for the HBT [31]. After glucose ingestion, breath samples were collected at 30-min intervals over a 2-h period. A positive HBT was defined as a 12 parts per million (ppm) increase in hydrogen concentration between peak and basal timepoints, within two hours of glucose ingestion. Clinically, an increase in HBT of ≥ 12 ppm over basal values signified a positive diagnosis of SIBO [31].
Statistical Analysis
A sample size of at least 44 randomly selected subjects per group (88 subjects total) was required to ensure a power of 0.80 at a 5% significance level for the comparison of safety and efficacy between the XG + PP and simethicone groups.
Results were presented using descriptive statistics: mean ± standard deviation (SD) for continuous variables and absolute (n) and relative (percent) for categorical variables.
Exploratory statistical tests were performed to determine possible differences in clinical signs and symptoms of FABD between the two groups. To assess whether the therapy was successful in alleviating the symptoms of FABD, a paired t test was initially performed to measure differences between baseline and different time points (research visits) in the entire sample, without subdivision between study arms. Exploratory statistical tests were then performed to determine whether the treatments differed in their ability to reduce symptoms of FABD. After normality assessment, t tests, Mann–Whitney U, Δ2 and Wilcoxon signed-rank tests were performed. Clinical symptoms and signs of FABD were associated with variables such as age, sex, treatment dose, or other recorded variables. p < 0.05 was considered statistically significant. The software used was IBM SPSS.
Results
This study included a total of 88 patients randomly assigned to the XG + PP (n = 44) and simethicone (n = 44) groups between January and June 2020. Fifty-seven patients were female and thirty-one were male, and the mean age was 45.27 (± 11.46) years in the XG + PP group and 40.68 (± 12.01) in the simethicone group (p = 0.0733). The two study groups were homogeneous, and randomization did not result in significant differences in baseline data, as shown in Table 1. All patients completed the study and data were analyzed for safety and efficacy.
Primary Outcomes
No Adverse Events, Serious Adverse Events, or Serious Unexpected Severe Adverse Reactions were reported during this study, indicating that both treatments were safe and well tolerated. Vital signs measured before and after the study visits showed no statistically significant differences between the XG + PP and simethicone groups at day 0 (baseline) and day 20 (end of treatment).
Secondary Outcomes
Patient Self-Assessment of Symptoms
Bloating
On day 1, improvement was observed as early as 60 min after XG + PP administration.
Notably, within 120 min of XG + PP administration, patients reported significant symptom relief (p = 0.002), in contrast to patients taking simethicone (p = 0.1978). Compared to simethicone, the results of the T test for 2 independent means showed that XG + PP had significantly higher efficacy in reducing bloating symptoms, as early as day 2 (p = 0.0364) with a 77.5% symptoms reduction by day 8 (p = 0.0279).
Abdominal Pain
Patients reported subjective relief of abdominal pain within 60 min of taking XG + PP. On day 1, within 120 min of XG + PP administration, patients reported significant symptom relief (p = 0.0009), in contrast to patients taking simethicone (p = 0.1770). Compared to simethicone, two independent means T test results show that XG + PP was significantly more effective in reducing abdominal pain within 120 min as early as day 2 (p = 0.0375). At day 8, abdominal pain was significantly reduced by 58.8% compared to simethicone within 120 min of XG + PP administration (p = 0.0094).
Distension
In contrast to simethicone, two independent means T test results showed that XG + PP was significantly superior at day 3 in reducing distension within 120 min of administration, (p = 0.0333) with efficacy significantly maintained up to day 8 (p = 0.0137).
Flatulence
On day 1, patients reported improvement in symptoms within 60 min of XG + PP administration. Notably, patients reported significant symptom relief within 120 min of XG + PP administration (p = 0.0018), in contrast to patients taking simethicone (p = 0.2149). While XG + PP and simethicone reduced flatulence by 61.2% and 29.2%, respectively, there was no statistically significant difference between the XG + PP and the simethicone groups from day 0 to day 8, before and after administration (60 and 120 min).
The self-assessed measurement results for the four variables during the eight consecutive days of surveys are shown in Fig. 1.
Self-assessed measurement results for the four variables: (i) Bloating, (ii) Abdominal pain, (iii) Flatulence, (iv) Distension. Values (Mean ± SD) were expressed according to the Visual Analogue Scale (VAS; y-axis). Each graph shows the changes observed on the eight consecutive days of the surveys and at the three time points considered for each measurement on each day. Gray bars indicate patients treated with simethicone; black bars indicate patients treated with XG + PP (Xyloglucan and Pea protein). Asterisks (*) indicate significant difference (significance at 5%) between XG + PP and simethicone groups
Figure 2 shows the changes observed for each variable on day 1 at the three time points considered in the study, i.e., before, after 60 min, and after 120 min.
Self-assessed measurement for the four variables: (i) Bloating, (ii) Abdominal pain, (iii) Flatulence, (iv) Distension. Values (Mean ± SD) are expressed according to the Visual Analogue Scale (VAS; y-axis). The graph shows the changes observed for each variable on day 1 at the three time points included in the study, i.e., before, 60 min, 120 min. Gray bars indicate patients treated with simethicone; black bars indicate patients treated with XG + PP (Xyloglucan and Pea protein). Asterisks (*) indicate significant difference (significance at 5%) in VAS values between XG + PP before the administration and after 120 min. No significant differences were observed when simethicone was considered
Abdominal Circumference
Subjects in the XG + PP and simethicone groups showed a reduction in abdominal circumference from day 0 (XG + PP mean: 95.50 ± 18.44 cm; simethicone mean: 92.59 ± 15.50 cm), to day 20 (XG + PP mean: 90.80 ± 14.09 cm; simethicone mean: 90.75 ± 15.23 cm). At day 20, abdominal circumference decreased by an average of 4.7 cm for XG + PP compared to 1.8 cm for simethicone. No statistically significant differences were observed between the XG + PP and the simethicone groups (p = 0.6169).
SIBO
Patients taking XG + PP for 20 days tested negative for SIBO at the end of the study (only 3 patients still had a positive diagnosis for SIBO). On day 0, a change in peak-basal HBT of 13.21 was observed in the XG + PP arm, indicating a positive diagnosis of SIBO. On day 20, a significant change in peak-basal HBT of 5.98 was observed, indicating that SIBO was no longer present (p = 0.00001, Table 2). On day 0, patients in the simethicone arm were diagnosed with SIBO (∆peak-basal HBT of 12.64) with no significant changes reported on day 20 (∆peak-basal HBT of 11.51) (23 patients had still a positive diagnosis for SIBO).
Discussion and Conclusions
Functional abdominal bloating and distension (FABD) affects patients’ quality of life. Treatment is often challenging because this condition is caused by multiple factors and can become chronic if not adequately treated [16]. The findings reported here are of remarkable clinical significance, considering that FABD is a very common gastrointestinal disorder worldwide [1,2,3, 32]. When choosing the most appropriate therapeutic approach, rapid action and safety are essential aspects to consider, as FABD patients usually require long-term treatment. This study shows that both treatments are well tolerated, as no adverse effects, serious adverse events, or serious unexpected adverse reactions were reported by patients or investigators during the study, as shown by the observation of normal vital signs at baseline and follow-up. These results show that the safety profile of XG + PP is comparable to that of simethicone.
Here, XG + PP demonstrated a faster onset of action compared to simethicone in reducing bloating, abdominal pain, and flatulence. Moreover, only patients treated with XG + PP tested negative for SIBO, a known cause of functional abdominal bloating and distension. Interestingly, a greater decrease in abdominal circumference was observed in the XG + PP group than in the simethicone arm.
Taken together, these results are particularly relevant given that patients suffering from FABD seek rapid symptom relief, making XG + PP an attractive alternative solution to standard treatments. This is confirmed by the results of the HBT test which show a statistically significant difference in the XG + PP group at day 0 compared to day 20.
Our results are consistent with previous studies showing symptoms improvement in patients with various gastrointestinal disorders treated with XG-containing products [30, 33,34,35,36]. Interestingly, de los Rios et al. [37] analyzed a cohort of 50 subjects with irritable bowel syndrome and demonstrated that a treatment containing XG and PP was clinically effective and safe in the long term, with a sustained response over the duration of therapy. The therapeutic benefit of XG + PP in preventing FABD was also demonstrated in an in vivo partial restraint stress model in which the product reduced visceral hypersensitivity and intestinal permeability [18].
XG + PP acts by creating a film-forming barrier that leads to a reduction of Small Intestinal Bacterial Overgrowth (SIBO) and FABD symptoms. SIBO is characterized by an excessively high bacterial population of more than 103 organisms/ml [38]. Bacterial overgrowth feeds on the undigested food in the small intestine and causes the fermentation of sugars and bile acids, producing hydrogen as a by-product. Hydrogen, in turn, is used by hydrogenotrophic archaea and bacteria to release methane and hydrogen sulfide, respectively, into the intestinal lumen [39]. In this context, XG has been shown to act as a physical barrier thanks to its “mucin-like” molecular structure and optimal mucoadhesive properties. In fact, XG has been shown to be able to protect the integrity of mucosal cells from various damaging agents, such as microorganisms, allergens and pro-inflammatory compounds [28, 40]. Moreover, XG and PP have been shown to synergistically create a protective mechanical barrier over intestinal epithelial cells that improves the architecture of intestinal tissues, restores the physiological barrier property of the epithelia, and prevents adhesion and proliferation of gas-producing coliforms [18, 30]. The present results contribute to support the importance of using mucomimetic agents to treat visceral hypersensitivity in colorectal distension, as they are effective and safe. In this context, Collins et al. pointed out that disruption of the balance between host and intestinal microbiota induces a range of changes in the mucosal immune system, from microscopic modifications to excessive inflammation, leading to alterations in gut sensory-motor function and immune activity [41].
Based on these findings, XG + PP may represent a useful etiology-based strategy for the treatment of gastrointestinal disorders by allowing the creation of a protective barrier that prevents the adhesion and proliferation of intestinal bacteria typical of SIBO, promotes the restoration of the integrity and functionality of the mucosal barrier, and helps the regulation of nutrient sensing. In contrast, simethicone acts via a completely different mechanism, decreasing the surface tension of gas bubbles in the gastrointestinal tract and facilitating their elimination, but is not effective in the treatment of SIBO [42]. Although few studies are currently available, they support the role of XG + PP in controlling bloating symptoms by regulating intestinal motility, gut flora, and visceral sensitivity.
These results are promising and highlight the importance of conducting larger studies with longer follow-up to confirm these preliminary findings. Because XG + PP addresses the root causes of the disease, its long-term efficacy may be greater than that of simethicone. Furthermore, the safety profile could be favorable for continued treatment, as XG + PP does not act through a pharmacological mechanism but simply works by mechanically protecting the intestinal mucosa. Given the efficacy of the product in treating bloating, we can consider XG + PP as a suitable therapeutic for patients suffering from general bloating. It would be interesting to investigate other target populations such as patients with common bloating disorders. There are many plausible causes of abdominal bloating, and accurate diagnosis is not always possible. Thus, effective non-pharmacological treatment with a high safety profile may be appropriate even in the absence of a diagnosis.
XG + PP may also be useful for certain patient populations, such as pregnant women or the elderly, for whom drug treatment is not recommended. Although simethicone is a long-used agent, it has some limitations in terms of its mechanism of action, so there is an urgent need to find effective, safer alternatives [23,24,25].
In summary, this preliminary study provides evidence that FABD patients may benefit from XG + PP-based treatment because it is safe and has a faster onset of action compared to simethicone.
Change history
02 April 2024
A Correction to this paper has been published: https://doi.org/10.1007/s10620-024-08378-w
References
Drossman DA, Li Z, Andruzzi E et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–1580.
Sperber AD, Bangdiwala SI, Drossman DA et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99-114.e3.
Jiang X, Locke GR 3rd, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756–763.
Mearin F, Lacy BE, Chang L et al. Bowel Disorders. Gastroenterology. 2016;S0016–5085:00222–00225.
Mari A, Abu Backer F, Mahamid M et al. Bloating and Abdominal Distension: Clinical Approach and Management. Adv Ther. 2019;36:1075–1084.
Lewis MJ, Reilly B, Houghton LA, Whorwell PJ. Ambulatory abdominal inductance plethysmography: towards objective assessment of abdominal distension in irritable bowel syndrome. Gut. 2001;48:216–220.
Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders – epidemiology and exploration of mechanisms. Aliment Pharmacol Ther. 2008;27:2–10.
Agrawal A, Houghton LA, Lea R, Morris J, Reilly B, Whorwell PJ. Bloating and distention in irritable bowel syndrome: the role of visceral sensation. Gastroenterology. 2008;134:1882–1889.
Malagelada JR, Accarino A, Azpiroz F. Bloating and Abdominal Distension: Old Misconceptions and Current Knowledge. Am J Gastroenterol. 2017;112:1221–1231.
Villoria A, Azpiroz F, Burri E, Cisternas D, Soldevilla A, Malagelada JR. Abdomino-phrenic dyssynergia in patients with abdominal bloating and distension [published correction appears in Am J Gastroenterol. 2011 Jul; 106(7):1405]. Am J Gastroenterol 2011;106:815–819.
Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada JR. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology. 2009;136:1544–1551.
Hernando-Harder AC, Serra J, Azpiroz F, Milà M, Aguadé S, Malagelada C et al. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol. 2010;105:876–882.
Shim L, Prott G, Hansen RD, Simmons LE, Kellow JE, Malcolm A. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010;105:883–887.
Noh CK, Lee KJ. Fecal Microbiota Alterations and Small Intestinal Bacterial Overgrowth in Functional Abdominal Bloating/Distention. J Neurogastroenterol Motil. 2020;26:539–549.
Ringel-Kulka T, Benson AK, Carroll IM, Kim J, Legge RM, Ringel Y. Molecular characterization of the intestinal microbiota in patients with and without abdominal bloating. Am J Physiol Gastrointest Liver Physiol. 2016;310:G417–G426.
Lacy BE, Cangemi D, Vazquez-Roque M. Management of Chronic Abdominal Distension and Bloating. Clin Gastroenterol Hepatol. 2021;19:219-231.e1.
Saffouri GB, Shields-Cutler RR, Chen J et al. Small intestinal microbial dysbiosis underlies symptoms associated with functional gastrointestinal disorders. Nat Commun. 2019;10:2012.
Scuderi SA, Casili G, Lanza M et al. Efficacy of a product containing Xyloglucan and Pea Protein on intestinal barrier function in a partial restraint stress animal model. Int J Mol Sci. 2022;23:2269.
Tuteja AK, Talley NJ, Joos SK et al. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008;103:1241–1248.
Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distension in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–1010.
Sullivan SN. Functional abdominal bloating with distension. ISRN Gastroenterol. 2012;2012:721820.
Pimentel M, Saad RJ, Long MD, Rao SSC. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am J Gastroenterol. 2020;115:165–178.
Liu JH, Chen GH, Yeh HZ, Huang CK, Poon SK. Enteric-coated peppermint-oil capsules in the treatment of irritable bowel syndrome: a prospective, randomized trial. J Gastroenterol. 1997;32:765–768.
Cappello G, Spezzaferro M, Grossi L, Manzoli L, Marzio L. Peppermint oil (Mintoil) in the treatment of irritable bowel syndrome: a prospective double-blind placebo-controlled randomized trial. Dig Liver Dis. 2007;39:530–536.
Tominaga K, Sakata Y, Kusunoki H et al. Rikkunshito simultaneously improves dyspepsia correlated with anxiety in patients with functional dyspepsia: A randomized clinical trial (the DREAM study). Neurogastroenterol Motil. 2018;30:e13319.
Ebadi M. Desk Reference of Clinical Pharmacology, 2nd edn. Boca Raton, FL, USA: CRC Press; 2011.
Bernstein JE, Kasich AM. A double-blind trial of simethicone in functional disease of the upper gastrointestinal tract. J Clin Pharmacol. 1974;14:617–623.
Piqué N, Gómez-Guillén MDC, Montero MP. Xyloglucan, a Plant Polymer with Barrier Protective Properties over the Mucous Membranes: An Overview. Int J Mol Sci. 2018;19:673.
Eckardt NA. Role of xyloglucan in primary cell walls. Plant Cell. 2008;20:1421–1422.
Trifan A, Burta O, Tiuca N, Petrisor DC, Lenghel A, Santos J. Efficacy and safety of Gelsectan for diarrhoea predominant irritable bowel syndrome: A randomised, crossover clinical trial. United European Gastroenterol J. 2019;7:1093–1101.
Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29 Suppl 1:1–49. Erratum in: Aliment Pharmacol Ther. 2010 Jan;31(1):166. Satta PU [corrected to Usai-Satta P].
Seo AY, Kim N, Oh DH. Abdominal bloating: pathophysiology and treatment. J Neurogastroenterol Motil. 2013;19:433–453.
Tao Y, Huang C, Lai C, Huang C, Yong Q. Biomimetic galactomannan/bentonite/graphene oxide film with superior mechanical and fire retardant properties by borate cross-linking. Carbohydr Polym. 2020;245:116508.
Pleșea Condratovici C, Bacarea V, Piqué N. Xyloglucan for the Treatment of Acute Gastroenteritis inChildren: Results of a Randomized, Controlled, Clinical Trial. Gastroenterol Res Pract. 2016;2016:6874207.
Esposito E, Campolo M, Casili G et al. Protective Effects of Xyloglucan in Association with the Polysaccharide Gelose in an Experimental Model of Gastroenteritis and Urinary Tract Infections. Int J Mol Sci. 2018;19:1844.
Periasamy S, Lin CH, Nagarajan B, Sankaranarayanan NV, Desai UR, Liu MY. Mucoadhesive role of tamarind xyloglucan on inflammation attenuates ulcerative colitis. J Funct Foods. 2018;47:1–10.
de Los Rios CC, Falcón BS, Arguelles-Arias F et al. Long-term safety and efficacy study of a medical device containing xyloglucan, pea protein reticulated with tannins and xylo-oligosaccharides, in patients with diarrhoea-predominant irritable bowel syndrome. Therap Adv Gastroenterol. 2021;14:17562848211020570.
Bushyhead D, Quigley EMM. Small Intestinal Bacterial Overgrowth-Pathophysiology and Its Implications for Definition and Management. Gastroenterology. 2022;163:593–607.
Carbonero F, Benefiel AC, Gaskins HR. Contributions of the microbial hydrogen economy to colonic homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:504–518.
Gnessi L, Bacarea V, Marusteri M, Piqué N. Xyloglucan for the treatment of acute diarrhea: results of a randomized, controlled, open-label, parallel group, multicentre, national clinical trial. BMC Gastroenterol. 2015;15:153.
Collins SM, Denou E, Verdu EF, Bercik P. The putative role of the intestinal microbiota in the irritable bowel syndrome. Dig Liver Dis. 2009;41:850–853.
Martínez-Vázquez MA, Vázquez-Elizondo G, González-González JA, Gutiérrez-Udave R, Maldonado-Garza HJ, Bosques-Padilla FJ. Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: systematic review and meta-analysis. Rev Gastroenterol Mex. 2012;77:82–90.
Acknowledgments
The authors would like to thank all patients participating in the study, as well as their caregivers, care teams, investigators, and research personnel at participating institutions. The authors thank CEBIS International for publication support. The study was sponsored by DEVINTEC SAGL in accordance with Good Publication Practice guidelines.
Funding
The study was sponsored by Devintec Sagl.
Author information
Authors and Affiliations
Contributions
CP, ZE, KE, and ED provided data acquisition; JS provided critical revision. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest directly relevant to this study. Javier Santos has served as consultant for Noventure SL, Devintec pharma, Reckitt, Ipsen, & Pileje and discloses present and past recent scientific collaborations with Salvat, Norgine, Alfa-Sigma, Cosmo, Adare, Ordesa, and Danone that do not constitute a conflict of interest in developing the content of the present manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Petrisor, D.C., Etropolska, Z., Elenski, K. et al. Efficacy and Safety of Pea Protein and Xyloglucan Versus Simethicone in Functional Abdominal Bloating and Distension. Dig Dis Sci 69, 161–168 (2024). https://doi.org/10.1007/s10620-023-08155-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08155-1