Abstract
Background
Recent epidemiological studies suggested correlation between gastric cancer (GC) and periodontal disease.
Aims
We aim to clarify involvement of lipopolysaccharide of Porphyromonas gingivalis (Pg.), one of the red complex periodontal pathogens, in the GC development.
Methods
To evaluate barrier function of background mucosa against the stimulations, we applied biopsy samples from 76 patients with GC using a Ussing chamber system (UCs). K19-Wnt1/C2mE transgenic (Gan) mice and human GC cell-lines ± THP1-derived macrophage was applied to investigate the role of Pg. lipopolysaccharide in inflammation-associated carcinogenesis.
Results
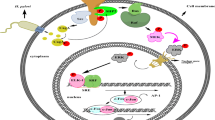
In the UCs, Pg. lipopolysaccharide reduced the impedance of metaplastic and inflamed mucosa with increases in mRNA expression of toll-like receptor (TLR) 2, tumor necrosis factor (TNF) α, and apoptotic markers. In vitro, Pg. lipopolysaccharide promoted reactive oxidative stress (ROS)-related apoptosis as well as activated TLR2-β-catenin-signaling on MKN7, and it increased the TNFα production on macrophages, respectively. TNFα alone activated TLR2-β-catenin-signaling in MKN7, while it further increased ROS and TNFα in macrophages. Under coculture with macrophages isolated after stimulation with Pg. lipopolysaccharide, β-catenin-signaling in MKN7 was activated with an increase in supernatant TNFα concentration, both of which were decreased by adding a TNFα neutralization antibody into the supernatant. In Gan mice with 15-week oral administration of Pg. lipopolysaccharide, tumor enlargement with β-catenin-signaling activation were observed with an increase in TNFα with macrophage infiltration.
Conclusions
Local exposure of Pg. lipopolysaccharide may increase ROS on premalignant gastric mucosa to induce apoptosis-associated barrier dysfunction and to secrete TNFα from activated macrophages, and both stimulation of Pg. lipopolysaccharide and TNFα might activate TLR2-β-catenin-signaling in GC.
Graphical Abstract






Similar content being viewed by others
Abbreviations
- LPS:
-
Lipopolysaccharide
- mUCs:
-
mini-Ussing chamber system
- NAC:
-
N-acetyl-l-cysteine
- Pg.:
-
Porphyromonas gingivalis
- PG:
-
Peptidoglycan
- uSS:
-
Updated Sydney system
- qPCR:
-
Quantitative polymerase chain reaction
- ROS:
-
Reactive oxygen species
- shRNA:
-
Short hairpin RNA
- TLR:
-
Toll-like receptor
- TNFα:
-
Tumor necrosis factor α
- TUNEL:
-
TdT-mediated dUTP nick end labeling
- HRP:
-
Horse radish peroxidase
- GC:
-
Gastric cancer
- H. pylori :
-
Helicobacter pylori
- post-eradication GC:
-
Gastric cancer after eradication treatment of H. pylori
- PMA:
-
Phorbol 12-myristate 13-acetate
- Mφ:
-
Macrophages
- Gan mice:
-
K19-Wnt1/C2mE transgenic mice
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
References
Graham DY. Helicobacter pylori infection is the primary cause of gastric cancer. J Gastroenterol. 2000;12:90–97.
Uemura N, Okamoto S, Yamamoto S et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789.
Sepulveda AR, Graham DY. Role of Helicobacter pylori in gastric carcinogenesis. Gastroenterol Clin N Am. 2002;31:517–535.
Brenner H, Arndt V, Stegmaier C et al. Is Helicobacter pylori infection a necessary condition for noncardia gastric cancer? Am J Epidemiol. 2004;159:252–258.
Amieva M, Peek RM. Pathobiology of Helicobacter pylori-induced gastric cancer. Gastroenterology. 2016;150:64–78.
Take S, Mizuno M, Ishiki K et al. Risk of gastric cancer in the second decade of follow-up after Helicobacter pylori eradication. J Gastroenterol. 2020;55:281–288.
Yan L, Chen Y, Chen F et al. Effect of Helicobacter pylori eradication on gastric cancer prevention: updated report from a randomized controlled trial with 26.5 years of follow-up. Gastroenterology. 2022;163:154–162.
Iijima K, Koike T, Abe Y et al. Alteration of correlation between serum pepsinogen concentrations and gastric acid secretion after H. pylori eradication. J Gastroenterol. 2009;44:819–825.
Kodama M, Murakami K, Okimoto T et al. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol. 2012;47:394–403.
Tari A, Kitadai Y, Sumii M et al. Basis of decreased risk of gastric cancer in severe atrophic gastritis with eradication of Helicobacter pylori. Dig Dis Sci. 2007;52:232–239.
Sekine H, Iijima K, Koike T et al. Regional differences in the recovery of gastric acid secretion after Helicobacter pylori eradication: evaluations with Congo red chromoendoscopy. Gastrointest Endosc. 2006;64:678–685.
Li TH, Qin Y, Sham PC, Lau KS, Chu KM, Leung WK. Alterations in gastric microbiota after H. pylori eradication and in different histological stages of gastric carcinogenesis. Sci Rep. 2017;7:44935.
Sung JJY, Coker OO, Chu E et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut. 2020;69:1572–1580.
Lo C-H, Kwon S, Wang L et al. Periodontal disease, tooth loss, and risk of oesophageal and gastric adenocarcinoma: a prospective study. Gut. 2021;70:620–621.
Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490.
Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63.
Uno K, Kato K, Atsumi T et al. Toll-like receptor (TLR) 2 induced through TLR4 signaling initiated by Helicobacter pylori cooperatively amplifies iNOS induction in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1004-1012.
Cadamuro ACT, Rossi AFT, Matos Biselli-Périco J et al. Effect of Helicobacter pylori eradication on TLR2 and TLR4 expression in patients with gastric lesions. Mediat Inflamm. 2015;2015:481972.
Ogawa T, Asai Y, Makimura Y, Tamai R. Chemical structure and immunobiological activity of Porphyromonas gingivalis lipid A. Front Biosci. 2007;12:3795–3812.
Darveau RP, Pham TT, Lemley K et al. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both Toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–5051.
Tye H, Kennedy CL, Najdovska M et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell. 2012;22:466–478.
West AC, Tang K, Tye H et al. Identification of a TLR2-regulated gene signature associated with tumor cell growth in gastric cancer. Oncogene. 2017;36:5134–5144.
Oshima H, Hioki K, Popivanova BK et al. Prostaglandin E2 signaling and bacterial infection recruit tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 2011;140:596–607.
Oshima H, Matsunaga A, Fujimura T et al. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095.
Zhou Q, Desta T, Fenton M, Graves DT, Amar S. Cytokine profiling of macrophages exposed to Porphyromonas gingivalis, its lipopolysaccharide, or its FimA protein. Infect Immun. 2005;73:935–943.
Holden JA, Attard TJ, Laughton KM, Mansell A, O’Brien-Simpson NM, Reynolds EC. Porphyromonas gingivalis lipopolysaccharide weakly activates M1 and M2 polarized mouse macrophages but induces inflammatory cytokines. Infect Immun. 2014;82:4190–4203.
Kondo S, Miyake M. Simultaneous prediction of intestinal absorption and metabolism using the mini-Ussing chamber system. J Pharm Sci. 2019;108:763–769.
Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181.
Bertazza L, Mocellin S. The dual role of tumor necrosis factor in cancer biology. Curr Med Chem 2010;17:3337–3352.
Liu Z, Brooks RS, Ciappio ED et al. Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem. 2012;23:1207–1213.
Guo C, Kim SJ, Frederick AM et al. Genetic ablation of tumor necrosis factor-alpha attenuates the promoted colonic Wnt signaling in high fat diet-induced obese mice. J Nutr Biochem. 2020;77:108302.
Kim J, Park C, Kim KH et al. Single-cell analysis of gastric pre-cancerous and cancer lesions reveals cell lineage diversity and intratumoral heterogeneity. npj Precis Oncol. 2022;6:9.
Tsuboi A, Ohsawa S, Umetsu D et al. Competition for space is controlled by apoptosis-induced change of local epithelial topology. Curr Biol. 2018;28:2115–2128.
Salazar CR, Francois F, Li Y et al. Association between oral health and gastric precancerous lesions. Carcinogenesis. 2012;33:399–403.
Iwashita M, Hayashi M, Nishimura Y, Yamashita A. The link between periodontal inflammation and obesity. Curr Oral Health Rep. 2021;8:76–83.
Mulhall H, Huck O, Amar S. Porphyromonas gingivalis, a long-range pathogen: systemic impact and therapeutic implications. Microorganisms. 2020;8:869.
Kato T, Yamazaki K, Nakajima M et al. Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere. 2018;3:e00460-18.
Arimatsu K, Yamada H, Miyazawa H et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep. 2014;4:4828.
Kageyama S, Takeshita T, Takeuchi K et al. Characteristics of the salivary microbiota in patients with various digestive tract cancers. Front Microbiol. 2019;10:1780.
Yuan X, Liu Y, Kong J et al. Different frequencies of Porphyromonas gingivalis infection in cancers of the upper digestive tract. Cancer Lett. 2017;404:1–7.
Sano M, Uchida T, Igarashi M et al. Increase in the lipopolysaccharide activity and accumulation of gram-negative bacteria in the stomach with low acidity. Clin Transl Gastroenterol. 2020;11:e00190.
Zhang D, Chen L, Li S, Gu Z, Yan J. Lipopolysaccharide of Porphyromonas gingivalis induces IL-1β, TNF-α and IL-6 production by THP-1 cells in a way different from that of Escherichia coli LPS. Innate Immun. 2008;14:99–107.
Mandell L, Moran AP, Cocchiarella A et al. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via Toll-like receptor 2 but not Toll-like receptor 4. Infect Immun. 2004;72:6446–6454.
Zhao DR, Jiang YS, Sun JY, Li HH, Luo XL, Zhao MM. Anti-inflammatory mechanism involved in 4-ethylguaiacol-mediated inhibition of LPS-Induced inflammation in THP-1 cells. J Agric Food Chem. 2019;67:1230–1243.
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661.
Arcone R, Palma M, Pagliara V, Graziani G, Masullo M, Nardone G. Green tea polyphenols affect invasiveness of human gastric MKN-28 cells by inhibition of LPS or TNF-α induced Matrix Metalloproteinase-9/2. Biochim Open. 2016;3:56–63.
Oguma K, Oshima H, Aoki M et al. Activated macrophages promote Wnt signaling through tumor necrosis factor-alpha in gastric tumor cells. EMBO J. 2008;27:1671–1681.
Echizen K, Horiuchi K, Aoki Y et al. NFκB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells. Oncogene. 2019;38:4250–4263.
Brenneman KE, Willingham C, Kilbourne JA, Curtiss R 3rd, Roland KL. A low gastric pH mouse model to evaluate live attenuated bacterial vaccines. PLoS ONE. 2014;29:e87411.
Acknowledgments
We thank Biomedical Research Unit of Tohoku University Hospital for technical support, and we thank the Cancer Research Institute of Kanazawa University for kind gift of K19-Wnt1/C2mE mice.
Funding
This work was supported by JSPS KAKENHI grant number 19K08434, and the grant for Basic Research of the Japanese Society for Helicobacter research (2020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 3813 kb)
Supplementary Figure. Comprehensive analysis of h-Pg. LPS-stimulated apoptosis-related gene expressions in MKN7. The apoptosis antibody array demonstrated the induction of the proteins of apoptosis-signaling and TNF-signaling in MKN7 (N = 2: Int/pos = the ratio of signal of the target gene per those of positive control).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oriuchi, M., Lee, S., Uno, K. et al. Porphyromonas gingivalis Lipopolysaccharide Damages Mucosal Barrier to Promote Gastritis-Associated Carcinogenesis. Dig Dis Sci 69, 95–111 (2024). https://doi.org/10.1007/s10620-023-08142-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08142-6