Abstract
Background
New Zealand (NZ) has one of the world’s highest rates of inflammatory bowel diseases (IBD), however available data are limited to southern, urban regions.
Aims
To determine the incidence and prevalence of IBD in the Manawatū region of NZ.
Methods
Patients in the Manawatū region, with a diagnosis of IBD made between 2011 and 2015 were identified. Demographic, diagnostic and disease data were collected, fulfilment of diagnostic criteria was assessed, and incidence rates were calculated. Comparison of disease phenotype and observed diagnostic criteria was made between diagnosis and 12-months following diagnosis. All resident patients with a diagnosis of IBD current on 5 March 2013 were identified, and prevalence rates were calculated.
Results
The mean annual age-standardised incidence rates of UC, CD, and IBD were 10.2, 17.0, and 27.2 per 100,000. IBD incidence was highest among those of European ethnicity (24.8 per 100,000), followed by Asian (1.4), and Māori (1.1). IBD incidence in the urban population was 34.0 per 100,000 (95% CI 24.1–46.0) compared to the rural population of 5.6 (95% CI 0.4–22.4). The age-standardised point prevalence of UC, CD, and IBD on 5 March 2013 was 157.7, 231.8, and 397.9 per 100,000, respectively.
Conclusions
The incidence and prevalence of IBD in the Manawatū region are comparable to those reported in other Australasian studies. Incidence was lower in Māori, and in the rural population. Follow-up is required to identify any changes in incidence and phenotype, and whether rural residence remains protective.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) and Crohn’s disease (CD), the two inflammatory bowel diseases (IBD), are chronic inflammatory conditions of the gastrointestinal (GI) tract. Although similarities are seen between the symptoms and clinical features of UC and CD, each condition largely has a distinct presentation. In UC, mucosal inflammation affects the rectum and some or all of the colon in continuity [1], while in CD, inflammation may affect any part of the GI tract, distribution is typically patchy and separated by sections of normal mucosa, and transmural inflammation can give rise to abscesses, strictures, and fistulas [2].
Since the end of the twentieth century, IBD incidence rates in Western countries have largely plateaued [3], while rates are rising rapidly in newly industrialised countries [4]. Stable and rising incidence rates, combined with population growth and low mortality in IBD, are causing prevalence rates to rise, increasing demand on already stretched healthcare services. The direct costs associated with IBD are substantial, contributed to by anti-tumour necrosis factor therapies [5] and the introduction of expensive newer monoclonal antibody treatments [6]. Given the significant implications for healthcare provision, there is an ongoing need to identify trends associated with IBD incidence and prevalence.
New Zealand (NZ), a country in the southwestern Pacific Ocean, has one of the highest reported rates of IBD worldwide. This has been well documented in the Canterbury region of the South Island, where between 2004 and 2014, the age-standardised incidence of IBD increased from 24.9 to 39.5 per 100,000 [7], and between 1996 and 2015 the incidence of paediatric IBD increased fourfold [8]. To the best of our knowledge, only two other published studies have examined the incidence of IBD in North Island locations of NZ. The first in 1962 described the incidence of UC in the capital city of Wellington [9], and the second described the incidence of IBD between 1969 and 1978 in Auckland [10]. The Manawatū differs from these previously studied regions. It is an agricultural area of NZ, with 23.5% of the population living in a rural location. It also has a higher proportion of Māori, who make up 20.6% of the population. The deprivation indexFootnote 1 for the region is 6.6, compared to 5.4 nationally. The aims of this study were to determine the incidence and prevalence of IBD in the Manawatū region of the North Island, a region of NZ not previously studied, and to assess disease features and diagnostic criteria observed at diagnosis and 12-months following diagnosis.
Materials and Methods
Study Design
A retrospective analysis was performed to identify all patients within the study area with a new diagnosis of IBD from 1 January 2011 to 31 December 2015. The study also aimed to identify all patients with an existing diagnosis of IBD who were residing in the study area on 5 March 2013.
Study Area and Population
The Manawatū region, and its health service provider MidCentral District Health Board (MidCentral DHB), are located in the lower central North Island of NZ. Dividing the region are the Tararua and Ruahine Ranges, a geographical feature that may result in some patients residing east of the ranges and being managed in neighbouring DHBs. To ensure any such patients were not missed from the cohort, the study area was restricted to the Manawatū region west of the ranges (Fig. 1). At the 2013 Census the study area population was 145,761 comprising 3.4% of the total NZ population. Ethnicities were European 77.6%, Māori 17.1%, Asian 6.3%, Pacific Peoples 3.8% and Middle Eastern/Latin American/African 0.8%. The proportion of European and Māori were higher than NZ as a whole (74.0% and 14.9%, respectively), and the proportion of Asian, Pacific Peoples, and Middle Eastern/Latin American/African were lower (11.8%, 7.4%, and 1.2%, respectively). Domicile distributions were urban 80.3% and rural 19.7% in the study area population, compared to urban 83.9% and rural 16.1% nationally.
Diagnostic Criteria
Diagnostic criteria were based on the Lennard–Jones anatomic criteria for the diagnosis of CD and UC [11] and the European Crohn’s and Colitis Organisation (ECCO) consensus diagnostic criteria [1, 2]. The criteria to meet a diagnosis of IBD were presence of symptom chronicity, demonstration of chronic inflammation in the GI tract, exclusion of other diagnoses such as infective colitis or diverticular disease, and demonstration of the Lennard–Jones criteria (Fig. 2). Symptom chronicity was defined as abdominal pain, diarrhoea (increased frequency of loose bowel motions), or per rectal (PR) bleeding for at least 6 weeks. Chronic inflammation was defined as at least one tissue sample of the GI tract showing histologic features of chronic inflammation: crypt architectural distortion, plasma cell infiltrate, lymphocyte infiltrate, or granulomata. In the absence of histological evidence, chronic inflammation was defined as two instances of demonstration of inflammation separated by at least 6 weeks. Demonstration of inflammation could be made at endoscopy, at surgery, on imaging [computed tomography enterography (CTE), magnetic resonance enterography (MRE)], or by a faecal calprotectin test (> 50 μg/g).
The Lennard–Jones criteria for UC were the presence of continuous mucosal inflammation without granulomata, solely affecting the rectum, or affecting the rectum and some or all the colon in continuity with the rectum. The diagnostic criteria for CD were two or more of the following Lennard–Jones criteria: discontinuous distribution of inflammation in the GI tract (including rectal sparing), transmural inflammation (presence of fistula, abscess, or fissuring ulcer), fibrosis (presence of stricture, or of submucosal fibrosis on histological specimen), lymphoid aggregate in GI tissue, crypt architectural distortion in GI tissue, mucin retention in colonic tissue, non-caseating granulomata in tissue from the GI tract, or non-colonic disease location (upper GI tract, anal). Upper GI tract disease location was present if any of the following were present: gastric granulomata, inflammation in the duodenum or jejunum, or gastric ulceration not typical of reflux oesophagitis or peptic ulcer disease. The presence of granulomata was attributed greater diagnostic weight (× 2) than other diagnostic criteria, namely fulfilment of two Lennard–Jones criteria while all other features each fulfilled one criterion. A diagnosis of inflammatory bowel disease unclassified (IBDU) was precluded in view of the absence of a comprehensive disease definition and established diagnostic criteria.
Diagnosis Algorithm
An algorithm-determined diagnosis and diagnosis date were derived from data in the 6 weeks preceding the leading clinician diagnosis date, through to 12 months post-diagnosis. Instances of the minimum diagnostic criteria not being met, disagreement between the algorithmic diagnosis and the diagnosis assigned by the leading clinician, or of disagreement between the date of diagnosis assigned by the algorithm and the date of diagnosis assigned by the leading clinician, were reviewed on a case-by-case basis. Resolution of algorithmic disagreement with leading clinician diagnosis centred on the diagnostic criteria observed.
Inclusion Criteria
Incidence criteria were assignment of a diagnosis of IBD by a gastroenterologist or general surgeon between 1 January 2011 and 31 December 2015 and domicile in the study area at the time of diagnosis. Exclusion criteria were diagnosis of IBD outside the incidence timeframe, domicile outside the study area at the time of diagnosis, incidental finding in an asymptomatic patient, or an alternative diagnosis e.g. ischaemic colitis.
Prevalence criteria were assignment of a diagnosis of IBD prior to 6 March 2013, and domicile in the study area on 5 March 2013. Exclusion criteria were diagnosis of IBD after 5 March 2013, domicile outside the study area on 5 March 2013, or an alternative diagnosis.
Patient Identification
Patients were identified by searching events for IBD keywords within eight MidCentral DHB data repositories, from 1 January 2011 to 31 December 2015. The keywords and their potential misspellings were “inflammatory bowel,” “inflamatory bowel,” “crohn’s,” “crohns,” “chrons,” “chron’s,” “ulcerative,” “colitis,” “indeterminate,” “indeterminite,” “ileitis,” “ilitis,” “enteritis,” “fistula,” “adalimumab,” “humira,” “asacol,” “mesalasine,” “mesalazine,” “pentasa,” “pentaza,” “azathioprine,” “azoathioprine,” “azathiaoprine,” “azoathioprine,” “infliximab,” “mercaptopurine,” “methotrexate,” “sulfa*,” and “sulph*”.
Clinic and procedure letters from all public and private gastroenterology clinics and general surgery practices in the Manawatū region were searched solely by keyword, MidCentral DHB endoscopy data were searched by keyword and by diagnosis coding of Crohn’s or colitis, public and private radiology data were limited to cross-sectional abdominal and pelvic imaging and were searched by keyword, all public and private histology data were limited to the ileum and colon and searched by SNOMED inflammatory code (Supplementary Table 1), all public and private laboratory data were searched for testing of 6-thioguanine and thiopurine methyltransferase, the Medlab Central region wide prescription database were searched for adalimumab, azathioprine, mercaptopurine, mesalazine, and lastly, hospital admissions were searched for primary and secondary ICD codes for UC, CD, and IBDU (Supplementary Table 2).
Data Collection
Primary demographic data (date of birth, gender, and ethnicity) were collected from the MidCentral DHB patient management database (WebPAS) for all patients with a diagnosis of IBD. This database is routinely updated with Primary Health Organisation Enrolment Collection data: a national collection that holds Primary Healthcare System patient enrolment data.
For incident patients, additional demographic and diagnostic data were collected from clinic letters, procedure reports, and admission records. In the instance that data could not be obtained from electronic records, hard-copy case files were viewed. Additional demographic data comprised family history of IBD, smoking history, and domicile at date of diagnosis. Domicile data were coded in accordance with population counts from the 2013 Census and Statistics NZ classifications: large urban, 30,000–99,999 residents; medium urban, 10,000–29,999 residents; small urban, 1000–9999 residents; and rural < 1000 residents.
Diagnostic data comprised the leading clinician IBD diagnosis and date of diagnosis, pre- and 12 months post-diagnosis symptom history: abdominal pain, diarrhoea, PR bleeding, perianal fistula occurrence and atypical symptom occurrence. All IBD related endoscopy, radiology, and surgical procedures in the 6 weeks preceding diagnosis through to 12 months post-diagnosis were also reviewed and coded. The data collected were procedure date, procedure indication, location of macroscopic inflammation, stenosis presence and location, fistula presence and location, location of biopsies taken, and histological findings. Location of inflammation was coded as one or many of oesophagus, stomach, duodenum, jejunum, proximal ileum, distal ileum (distal 30 cm), caecum, right colon (proximal to the splenic flexure), left colon, sigmoid colon, rectum, and anus. The histological findings recorded were architectural distortion, chronic inflammatory cell infiltrate in the lamina propria, crypt abscess (> 1), granuloma (> 1), deep fissuring ulceration, preservation of goblet cells, transmural or submucosal fibrosis, viral cytopathic inclusions in endothelial cells, helicobacter pylori organisms, low- or high- grade dysplasia, Paneth cell metaplasia, and normal/unremarkable.
A portion of the study data reported involvement of the rectum and left colon without reference to the transverse colon or splenic flexure. Consequently, a clear distinction between left sided and extensive UC sometimes could not be made. In order to prevent an over-estimation of patients with extensive disease, a conservative approach was taken whereby classification of extensive UC was defined as involvement of the right colon, or of colon proximal to 60cm from the anus.
For all patients identified without a diagnosis of IBD, rudimentary demographic and diagnosis data were collected to prevent repeat identification and processing as different data sources were reviewed. Collected data were entered into a secure purpose-built Microsoft Access database.
Statistical Analysis
Data were analysed using JASP computer software (version 0.16.3.0). Categorical variables, including demographics and disease subtype, were assessed by Pearson’s chi-square test and reported as absolute value and percentage. Continuous variables, namely age at diagnosis and duration of disease, were assessed by T test and reported as the mean. Significance was set at p < 0.05.
Age was standardised using the World Health Organization’s World Standard Population Distribution [12]. The incidence 95% confidence intervals (CI) were calculated assuming a Poisson distribution. Incidence and prevalence were defined per 100,000 persons in the study area population.
Population data, and urban and rural population parameters, were obtained from Statistics New Zealand. Incident risk ratios (IRR) with 95% CI’s were calculated for domicile at date of diagnosis. Disease phenotype was described using the Montreal classification [13]. Algorithms to perform keyword searches, process raw data to identify cases, assign a date that minimum diagnostic criteria were met, and assign an algorithmic diagnosis of IBD, were performed in the R statistical computing environment (R Core Team, version 4.1.1).
For incident cases, the Montreal classification, procedure summary (endoscopy, radiology, surgery), and Lennard–Jones criteria, were determined at the time of diagnosis and 12-months post-diagnosis. Procedures at diagnosis were based upon data preceding the diagnosis date through to 3 days post-diagnosis. The Montreal classification and Lennard–Jones criteria at diagnosis were based upon data preceding the diagnosis date through to 30 days post-diagnosis to allow for possible procedure delays associated with high healthcare service demand. Observations at 12-month post-diagnosis were based upon subsequent data through to 365 days post-diagnosis date.
Results
Annual Incidence of IBD in the Manawatū Region
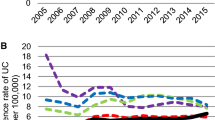
Between 1 January 2011 and 31 December 2015, 207 patients were diagnosed with IBD in the Manawatū region (UC 78, CD 129). The mean annual crude incidence rates per 100,000 were 10.7 (95% CI 6.5–17.6) for UC, 17.7 (95% CI 12.0–26.0) for CD, and 28.4 (95% CI 21.0–38.5) for IBD. Over the 5-year study period, the highest annual incidence for IBD was 35.0 (95% CI 26.6–46.0) per 100,000 in 2012, and the lowest was 19.9 (95% CI 13.8–28.6) per 100,000 in 2015. After standardising the data for age, the mean annual incidence rates for UC, CD, and IBD were 10.2, 17.0, and 27.2 per 100,000, respectively.
Demographics of the IBD Incident Cohort
Female IBD incident cases (n = 116, 56.0%) were greater than male (n = 91, 44.0%), with similar gender distribution for UC (n = 44, 56.4%) and CD (n = 72, 55.8%). Analysis of gender distribution by age at diagnosis or year of diagnosis revealed some instances of male predominance, although no trend with either variable was observed. Age at diagnosis followed a bimodal distribution for both UC and CD (Fig. 3). The primary peak in age was at 20–29 years, and a secondary peak at 40–49 years. The median age at diagnosis was 43.0 years for UC and 42.5 years for CD. Ethnicity was predominately European (n = 181, 87.4%), followed by Asian (n = 10, 4.8%), and Māori (n = 8, 3.9%). The minority ethnicities were African (n = 3, 1.4%), Latin American (n = 2, 1.0%), and Pacific Peoples (n = 1, 0.5%). Ethnicity data were unavailable for two patients.
A greater proportion of patients with UC (n = 12, 15.4%) reported a family history of IBD than patients with CD (n = 14, 10.9%). Patients reported only one (n = 21, 80.8%) or two (n = 5, 19.2%) family members with a diagnosis of IBD. Crohn’s disease (n = 22, 71.0%) was the most frequently reported form of IBD diagnosed in a family member. This was true in both patients with UC (n = 8, 61.5%) and patients with CD (n = 14, 77.8%). At the time of diagnosis, more than half of patients with CD were previous or current cigarette smokers (n = 66, 51.2%). The rate was lower in patients with UC (n = 33, 42.3%) (p = 0.261). The difference in current smoking rates was greater, twice as high in patients with CD (n = 20, 15.5%) compared to UC (n = 6, 7.7%) (p = 0.115), while the rates of previous smoker were similar: UC 32.1% and CD 34.1%.
The proportion of rural and urban residents did not differ between patients with UC and patients with CD. Eight cases of IBD were identified among rural residents (n = 28,707), and 199 among urban residents (n = 117,054). The mean annual rural and urban IBD incidence rates were 5.6 (95% CI 0.4–22.4) and 34.0 (95% CI 24.1–46.0) per 100,000, respectively. The incidence rate did not differ significantly between small, medium, or large urban area (31.4, 32.5, and 35.1 per 100,000, respectively). Urban residence (≥ 1000 residents) was a risk factor for CD (IRR 6.08, 95% CI 2.5–19.1, p < 0.001) and UC (IRR 6.13, 95% CI 2.0–30.4, p < 0.001).
Presenting Symptoms of the IBD Incident Cohort
The most frequently observed presenting symptom in patients with UC was PR bleeding (n = 67, 85.9%), and in patients with CD, diarrhoea (n = 104, 80.6%) (Table 1). Less common symptoms were observed in 12 (5.8%) patients. These were anal pain in one patient with UC (1.3%), and in patients with CD these were vomiting (n = 5, 3.9%), iron deficiency (ferritin < 30 ug/l) (n = 3, 2.3%), anal pain (n = 1, 0.8%), persistent gum lesion and lip swelling (n = 1, 0.8%) and mucus discharge and bloating (n = 1, 0.8%). A perianal fistula was observed in four (3.1%) patients with CD in the 6 months prior to the date of diagnosis.
Investigative Procedures Performed at Diagnosis and 12 Months Post-diagnosis
All 78 patients with UC, and 124 (96.1%) patients with CD, underwent diagnostic endoscopy, totalling 248 procedures (Table 2). 48 diagnostic imaging procedures were performed on seven (9.0%) patients with UC and 32 (24.8%) patients with CD. While eight (3.9%) patients, all diagnosed with CD, underwent one surgical procedure. At 12 months post-diagnosis, an additional 47 endoscopies, 91 imaging procedures, and 18 surgeries had been performed. Of the five patients with CD that did not undergo diagnostic endoscopy, three were diagnosed during surgical resection, one patient during rectal examination under anaesthetic (EUA), and one following radiological investigation.
Montreal Classification of the IBD Incident Cohort
Disease phenotype according to the Montreal Classification [13] is given in Table 3. In patients with UC, left-sided UC (E2) was observed most frequently at diagnosis (n = 38, 48.7%). Patients with E1 disease extent at diagnosis were significantly younger at diagnosis than those with E2 (32.6 v 45.5, p = 0.035) and E3 (32.6 v 46.0, p = 0.043). In patients with CD, the colon (L2) was the most common disease location at diagnosis (n = 57, 44.2%) and the majority (n = 111, 86.0%) had inflammatory (B1) disease behaviour. One (0.8%) patient had isolated upper GI disease, perianal disease was observed in six (4.7%) patients, and one of the four B3 patients also presented with stricturing disease behaviour. The mean diagnosis age of patients with L2 disease location was significantly greater than L1 (51.6 v 40.2, p = 0.01) and L3 (51.6 v 33.0, p < 0.001). There was also a nonsignificant trend for L3 patients to be younger than L1 patients (33.0 v 40.2, p = 0.09). Diagnosis age did not differ significantly by disease behaviour.
At 12 months post-diagnosis, disease extent remained stable in 76 (97.4%) patients with UC. The UC reclassifications included E1 to E2 (n = 1), and E2 to E3 (n = 1). Among patients with CD, disease location and behaviour remained stable in 123 (95.3%) and 120 (93.0%) of patients, respectively. Disease location reclassifications included L1 to L3 (n = 1) and L2 to L3 (n = 5), and disease behaviour reclassifications included B1 to B2 (n = 1), B1 to B3 (n = 3), and B2 to B3 (n = 3). Perianal location increased from six (4.7%) to ten (7.8%) patients, and seven (5.4%) of the ten B3 patients also presented with stricturing disease behaviour.
Symptom Chronicity, Chronic Inflammation and Lennard–Jones Diagnosis Criteria
Diagnosis criteria were assessed in the 219 patients with sufficient diagnostic data. 12 (5.5%) did not meet the minimum symptom duration of 6 weeks at the leading clinician diagnosis date. Following individual case review, four patients were excluded from the cohort: two instances of acute symptoms, and two of self-limiting colitis. Eight of these patients remained in the cohort. Three patients presented with acute symptom onset requiring admission: one with an inflammatory mass and retroperitoneal abscess, one with acute abdominal pain and a thickened terminal ileum on MRE, and one with acute abdominal pain who underwent subsequent hemicolectomy. Symptoms observed in the remaining five patients were: 4–5 weeks PR bleeding; 4–5 weeks abdominal pain, diarrhoea, and unintentional weight loss; chronic occasional diarrhoea, urgency and tummy upset, and chronic iron deficiency; 6 months perianal discomfort and excoriation; and 7 months of mucus discharge and bloating with a history of perianal abscesses.
Evidence of chronic inflammation (one histological instance, or two non-histological instances separated by 6 weeks) could not be demonstrated for eight of the remaining 215 patients. Specifically, two cases were of non-histological evidence separated by fewer than 6 weeks, four cases were of a sole instance of non-histological evidence, and two cases had no evidence of chronic inflammation. These eight patients were excluded from the cohort.
The required Lennard–Jones criteria were demonstrated in all 78 patients with UC at diagnosis (Table 4). At 12 months post-diagnosis, diagnostic criteria for CD were observed in three (3.8%) of these patients. Among the 129 patients with CD, five cases (2.4%) were reviewed on account of not meeting the minimum number of two Lennard–Jones CD criteria at the date of diagnosis. Non-colonic location was observed in four patients, and discontinuous distribution of inflammation in one patient. All five patients remained in the cohort.
Application of the Diagnosis Algorithm
The leading clinician diagnoses of the 207 confirmed patients were IBDU 15, UC 79, and CD 113. Application of the diagnosis algorithm identified 41 instances of disagreement with the leading clinician diagnosis (IBDU 15, UC 17, CD 10). A considered diagnosis of UC was assigned to six patients with a leading clinician diagnosis of IBDU, and to ten patients with a leading clinician diagnosis of CD. These changes were made on the basis of demonstrated continuous mucosal inflammation, rectal inflammation, and lymphoid aggregate. A considered diagnosis of CD was assigned to nine patients with a leading clinician diagnosis of IBDU, and 17 with a leading clinician diagnosis of UC. These changes were made on the basis of demonstrated lymphoid aggregate along with one or two of: non-colonic disease location, discontinuous distribution of inflammation, submucosal fibrosis, colonic mucin retention, or non-caseating granulomata.
IBD Incident Patient Identification and Diagnosis Confirmation
Keyword search within the eight data repositories identified 5146 unique patients (Supplementary Table 3). A total of 219 patients remained after exclusion of invalid National Health Index numbers, patients with no diagnosis or diagnosis other than IBD, patients diagnosed outside the study timeframe or outside the study area, incidental findings, and patients with insufficient data to confirm IBD diagnosis. The final incident cohort comprised 207 patients, 78 patients with UC and 129 with CD. The greatest number of incident cases were identified from histology data, followed by MidCentral endoscopy data, then Medlab Central prescription data.
Point Prevalence of IBD in the Manawatū Region
At March 2013, the crude prevalence rate of IBD per 100,000 in the Manawatū region was 456.9 (95% CI 423.6–492.9). The leading clinician diagnoses were IBDU 16, UC 285, and CD 365, equating to 11.0 (95% CI 6.7–17.9), 195.5 (95% CI 174.1–219.6), and 250.4 (95% CI 226.0–277.4) per 100,000, respectively. After age-standardisation the prevalence rates per 100,000 were 8.4 for IBDU, 157.7 for UC, 231.8 for CD, and 397.9 for IBD. Of the 666 patients identified with evidence of an IBD diagnosis, the greatest number of prevalence cases were identified from Medlab Central prescription data (n = 496), followed by MidCentral gastroenterology clinic data (n = 381), then MidCentral endoscopy data (n = 324).
Demographics of the IBD Prevalence Cohort
A greater proportion of patients with IBD were female (n = 359, 53.9%). Gender analysis by disease subtype demonstrated a significantly greater number of female patients with CD (211 vs 154, p = 0.003), while UC was slightly more common among males (51.6%).
The median age was 51.8 years for patients with IBD, 56.2 for UC, 47.2 for CD, and 59.9 for IBDU. Patients with UC were significantly older than CD (55.9 vs 48.2, p < 0.001). Prevalence rate was highest in the 60–69 years age group, followed by 40–49 years. Ethnicity was predominantly European (n = 620, 93.1%), followed by Māori (n = 21, 3.2%), and Asian (n = 12, 1.8%). The remaining ethnic groups each comprised less than 1% of the prevalent cohort.
The domicile of prevalence patients on 13 March 2013 was predominately small, medium or large urban (485, 651, and 513 per 100,000, respectively) with fewer patients residing in a rural area (94 per 100,000). Urban or rural residence did not differ between disease subtype (p = 0.392).
The date of IBD diagnosis was identified for 541 patients. In this group the median disease duration at March 2013 is 7.1 years for patients with IBD, 8.2 for UC, 6.9 for CD, and 1.8 for IBDU. Disease duration was significantly greater in patients with CD compared to IBDU (9.2 vs 2.5, p = 0.004), and UC compared to CD (11.6 vs 9.2, p = 0.005) and IBDU (11.6 vs 2.5, p < 0.001).
Discussion
The mean annual age-standardised incidence rates for UC, CD, and IBD were 10.2, 17.0, 27.2 per 100,000, respectively, from 2011 to 2015 in the Manawatū region. These rates correspond well to the 2004 Canterbury UC, CD, and IBD rates of 7.5, 16.3, and 25.2 per 100,000, respectively [14], as well as the 2012 UC, CD, and IBD rates of 6.3, 21.8, and 29.8 per 100,000, respectively, in the neighbouring Otago region [15]. Although, the follow-up Canterbury study revealed incidence rates 1.6-fold greater than 10 years earlier [7], and the identification methods employed in the Otago study may have resulted in underestimation of some cases [15], suggesting the incidence rates in the current study are lower than expected. One possible explanation is regional variability. Alternatively, this observation is in agreement with greater prevalence rates of paediatric IBD in the South Island compared with the North [16]. Given the geographical location of the South Island, and the existence of a north to south IBD gradient in the Northern Hemisphere, particularly for CD [17], this may be evidence of a south to north IBD gradient in the Southern Hemisphere.
In Australia, the IBD incidence rates reported from 2005 to 2011 almost mirror the rates seen in NZ. Two prospective population-based studies in Victoria reported annual IBD incidence rates of 29.3 (crude) per 100,000 between 2007 and 2008 [18], and 24.7 (age-standardised) per 100,000 between 2010 and 2011 [19]. In north Brisbane, Queensland, an earlier study in 2005 reported an IBD incidence of 30.3 per 100,000 [20]. Given the parallels between Australian and NZ, it is probable that genetic backgrounds and environmental risk factors are similar across both populations and largely explain the agreement between incidence rates.
Data on the prevalence of IBD is scarce, and rates may be underestimated due to difficulties associated with case identification and data acquisition. However, the observed IBD prevalence of 456.9 per 100,000 is higher than rates previously reported in NZ and Australia [14, 19]. In terms of global estimates, the highest reported prevalence rates are for North America and Europe: ranging from 340.0 to 505.0 per 100,000 for UC, and 262.0 to 322.0 per 100,000 for CD [3]. This places the observed prevalence of CD (250.4 per 100,000) alongside some of the highest reported rates worldwide.
Among prevalent cases, the ratio of CD:UC was 1.3:1, higher than the 1.1:1 seen in the 2004 Canterbury study [14], and both rates are lower than their corresponding CD:UC incidence ratios of 1.7:1 and 2.2:1, respectively. These findings align with the decrease or plateauing of UC incidence, and ongoing rise in CD incidence observed in many other westernised countries [3, 21]. In fact, recent findings suggest that the division between UC and CD rates in NZ may increase further. The research by Lopez et al. [16] demonstrated a CD:UC ratio of 5:1 among paediatric prevalent cases in 2015, while the incidence of paediatric IBD between 1996 and 2015 equates to a CD:UC ratio of 8.4:1 [8].
Urban residence at the time of diagnosis was associated with a sixfold greater incidence of IBD compared to rural residence. In a meta-analysis of 40 studies by Soon et al. [22], a positive association was found between living in an urban environment and risk of both CD and UC irrespective of disparities between urban population definitions, or even the absence of a definition. Similarly, in a large Canadian study, 14 different definitions of rural/urban were assessed and an association between rural residence and reduced risk of paediatric IBD persisted for most definitions [23]. The higher incidence of IBD in urban settings is thought to stem from increased hygiene, leading to a decrease in exposure to immune system priming and impairment of immunoregulatory mechanisms [24]. Whereas rural environments provide greater opportunity for microorganism exposure through contact with pets, livestock, soil, and untreated water. Another potentially contributing factor is an imbalance between urban and rural healthcare and implications for time to diagnosis. Correspondingly, in a recent study of NZ rural patients with IBD, time and financial constraints were identified as a significant challenge in accessing urban-based healthcare, and privacy concerns associated with living in smaller communities led to avoidance of rural IBD services [25].
Disease location in patients with UC was generally consistent with other reports for left-sided and extensive UC, while proctitis was seen in a small proportion of patients (n = 17, 21.8%) at diagnosis compared to 30–49% reported in other Australasian and European studies [7, 18, 19, 26, 27]. An unexpected finding was the significantly younger diagnosis age of patients with proctitis, contrary to reports in the Canterbury study and a large Swedish study with extensive UC being associated with a significantly younger diagnosis age [26, 28]. Disease extent was stable in the majority of patients with UC in the 12 months following diagnosis with only two (2.6%) reclassifications. In patients with CD, the proportion of ileal disease location (n = 30, 23.3%) was at the lower end of those seen in comparable studies, ileocolonic and upper GI disease were in accord, and a high proportion of patients had colonic disease (n = 57, 44.2%) [7, 15, 18, 19, 26, 27]. With regard to disease behaviour, the low rate of perianal disease (n = 6, 4.7%) was the sole atypical observation. Tarrant et al. [26] demonstrated that the presence of perianal disease can predict an enhanced risk of extensive and complicated disease progression. This was not evident in the current study as of the six location reclassifications, no patients had perianal disease, and of the nine behaviour reclassifications, only one patient had perianal disease. Ileal and upper GI disease has also been associated with a greater risk of disease progression [29, 30]. Instead, in this study disease progression was observed in patients with ileal, ileocolonic, and colonic disease. These different outcomes likely reflect the small cohort size and short follow-up period.
Early diagnosis and intervention markedly improve disease prognosis, yet the diagnostic definition of IBD (particularly for CD) remains vague, and usage of the few well-defined definitions is scant. Among the 219 patients with a leading clinician diagnosis of IBD, 25 patients (IBDU 4, UC 2, CD 16) did not meet the criteria for symptom chronicity, demonstration of chronic inflammation, or demonstration of Lennard–Jones criteria. This suggests such criteria are too rigid to diagnose mild or early-stage IBD, most notably CD. We believe a consensus is required on minimum diagnostic criteria that allow for earlier diagnosis of this patient subset. With regard to CD, most patients did not formally meet Lennard–Jones criteria at diagnosis due to a lack of demonstration of disease beyond the mucosa of the bowel. Diagnostic criteria that do not rely on demonstration of transmural involvement of bowel tissue would allow description of the natural history of early CD, and study of early therapeutic intervention. Until this is achieved, our knowledge of the management of CD is unfortunately limited to those who have already suffered transmural complications.
This study has several limitations. Firstly, the collection of some data was compromised by the retrospective study design. Specifically, demographic and diagnosis data could not be located for some prevalent cases which restricted the scope of analysis and may have led to the inclusion of cases where the diagnosis of IBD has since been revoked. Secondly, it is possible that some incident cases were not identified. Although we anticipate this number is low due to the inclusion of additional data sources that only record information for patients managed in the study area, specifically histology reports and prescription data. Similarly, it is possible that prevalent cases in remission for an extended period of time; managed without prescription of adalimumab, azathioprine, mesalazine or methotrexate; or managed outside the study area; were not identified. Thirdly, instances of sigmoidoscopy, or premature colonoscopy cessation, may have resulted in underrepresentation of UC extent and CD location. Study strengths include robust data collection through extensive utilisation of data repositories. Further, data in this study have been presented in an objective and detailed format. This will allow accurate comparison of this cohort against other cohorts. It will also allow accurate comparison of future cohorts, even if the diagnostic criteria for IBD change in the future.
In conclusion, in the Manawatū region the mean annual age-adjusted IBD incidence from 2011 to 2015 was 27.2 per 100,000, and the 2013 age-adjusted prevalence was 397.9 per 100,000. Rates of IBD were significantly reduced in the rural community, an observation that warrants further research. Comparison of findings between IBD cohorts in different locations is compromised by a lack of uniformity in assigning a diagnosis of IBD, usually at the mild end of the disease severity spectrum. We consider that an objective diagnostic definition of early stage IBD, particularly CD, is needed in order to reduce inter-observer variability, and to better standardise cohorts of patients with IBD.
Notes
Represents the areas with the least deprived scores and 10 represents the areas with the most deprived scores.
References
Magro F, Gionchetti P, Eliakim R et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670.
Gomollón F, Dignass A, Annese V et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25.
Ng SC, Shi HY, Hamidi N et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778.
Alatab S, Sepanlou SG, Ikuta K et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30.
van der Valk ME, Mangen MJ, Leenders M et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFα therapy: results from the COIN study. Gut. 2014;63:72–79.
Gisbert JP, Chaparro M. Predictors of primary response to biologic treatment [anti-TNF, vedolizumab, and ustekinumab] in patients with inflammatory bowel disease: from basic science to clinical practice. J Crohns Colitis. 2020;14:694–709.
Su HY, Gupta V, Day AS, Gearry RB. Rising incidence of inflammatory bowel disease in Canterbury, New Zealand. Inflamm Bowel Dis. 2016;22:2238–2244.
Lopez RN, Appleton L, Gearry RB, Day AS. Rising incidence of paediatric inflammatory bowel disease in Canterbury, New Zealand, 1996–2015. J Pediatr Gastroenterol Nutr. 2018;66:e45–e50.
Wigley RD, Maclaurin BP. A study of ulcerative colitis in New Zealand, showing a low incidence in Maoris. Br Med J. 1962;2:228–231.
Eason R, Lee S, Tasman-Jones C. Inflammatory bowel disease in Auckland, New Zealand. Aust N Z J Med. 1982;12:125–131.
Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol. 1989;24:2–6.
Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M. Age standardization of rates: a new WHO standard. Geneva World Heal Organ. 2001;9:1–14.
Silverberg MS, Satsangi J, Ahmad T et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19:5A-36A.
Gearry RB, Richardson A, Frampton CM et al. High incidence of Crohn’s disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis. 2006;12:936–943.
Coppell KJ, Galts CP, Huizing FY et al. Annual incidence and phenotypic presentation of IBD in southern New Zealand: an 18-year epidemiological analysis. Inflamm Intest Dis. 2018;3:32–39.
Lopez RN, Evans HM, Appleton L et al. Point prevalence of pediatric inflammatory bowel disease in New Zealand in 2015: initial results from the PINZ study. Inflamm Bowel Dis. 2017;23:1418–1424.
Schultz M, Butt AG. Is the north to south gradient in inflammatory bowel disease a global phenomenon? Expert Rev Gastroenterol Hepatol. 2012;6:445–447.
Wilson J, Hair C, Knight R et al. High incidence of inflammatory bowel disease in Australia: a prospective population-based Australian incidence study. Inflamm Bowel Dis. 2010;16:1550–1556.
Studd C, Cameron G, Beswick L et al. Never underestimate inflammatory bowel disease: high prevalence rates and confirmation of high incidence rates in Australia. J Gastroenterol Hepatol. 2016;31:81–86.
Hanigan K, Radford-Smith GL. The incidence of IBD in north Brisbane-a population study. J Gastroenterol Hepatol. 2008;23:A215.
Molodecky NA, Soon IS, Rabi DM et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.
Soon IS, Molodecky NA, Rabi DM, Ghali WA, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012;12:1–14.
Benchimol EI, Kaplan GG, Otley AR et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: a population-based inception and birth cohort study. Am J Gastroenterol. 2017;112:1412–1422.
Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5–15.
Richard L, Noller G, Derrett S et al. Patients’ accounts of living with and managing inflammatory bowel disease in rural Southern New Zealand: a qualitative study. BMJ Open. 2020;10:e041789.
Tarrant KM, Barclay ML, Frampton CM, Gearry RB. Perianal disease predicts changes in Crohn’s disease phenotype-results of a population-based study of inflammatory bowel disease phenotype. Am J Gastroenterol. 2008;103:3082–3093.
Burisch J. Crohn’s disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan Med J. 2014;61:B4778.
Sjöberg D, Holmström T, Larsson M et al. Incidence and natural history of ulcerative colitis in the Uppsala Region of Sweden 2005–2009—results from the IBD Cohort of the Uppsala Region (ICURE). J Crohns Colitis. 2013;7:e351–e357.
de Barros KSC, Flores C, Harlacher L, Francesconi CFM. Evolution of clinical behavior in Crohn’s disease: factors associated with complicated disease and surgery. Dig Dis Sci. 2017;62:2481–2488. https://doi.org/10.1007/s10620-017-4685-9.
Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155.
Acknowledgments
We would like to thank Bruce Lockett, Medlab Central; John Goulden, MidCentral DHB; Greg Bolton, MidCentral DHB; Peter Dixon, Broadway Radiology; Leigh Jewell, Broadway Radiology; Dr Nick Tindle, Mr Mike Young, Mr Chris Daynes, and Dr Ross Hayton for their assistance in data acquisition.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. Funding for this study was received from the Palmerston North Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. HM participated in data acquisition, analysis, and interpretation of data, and wrote the manuscript draft. JC and KCP critically revised the manuscript. JRI participated in data acquisition, analysis, and interpretation of data, supervised the study, and critically revised the manuscript. All authors have read and approved the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The study was approved by the Health and Disability Ethics Committees (ref 16/STH/218), MidCentral DHB (ID 2016.09.002) and MidCentral DHB Māori Research Committee. This study was performed in line with the principles of the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-023-08073-2.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Morton, H., Coad, J., Pedley, K.C. et al. Incidence of Inflammatory Bowel Disease in New Zealand Remains High, Findings in the Manawatū Region. Dig Dis Sci 68, 4230–4242 (2023). https://doi.org/10.1007/s10620-023-08070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-08070-5