Abstract
Background
Outpatient visits and laboratory assessments are routinely scheduled every 3 to 4 months in thiopurine-treated patients with inflammatory bowel disease (IBD) to timely detect thiopurine-related adverse events (AEs). AEs that require therapy adjustment beyond 12 months of treatment are rare.
Aim and Methods
This single-center prospective cohort study evaluated the safety of a reduced 6-monthly monitoring strategy in steroid-free patients with quiescent IBD on stable dose of azathioprine, mercaptopurine, or thioguanine monotherapy. The primary outcome was thiopurine-related AEs requiring therapy adjustments during a follow-up period of 24 months. Secondary outcomes included all AEs including laboratory toxicity, disease flares until 12 months, and the net monetary benefit from this strategy concerning IBD-related health care use.
Results
We enrolled 85 patients with IBD (median age 42 years, 61% Crohn’s disease, 62% female), with a median disease duration of 12.5 years and median thiopurine treatment duration of 6.7 years. During follow-up, 3 patients (4%) ceased thiopurines due to AEs: recurrent infections, non-melanoma skin cancer, and gastrointestinal complaints (nausea, vomiting). At 12 months, 25 laboratory toxicities were observed (including 13% myelotoxicity, 17% hepatotoxicity); none required therapy adjustments and all were transient. A reduced monitoring strategy had a net benefit of €136 per patient.
Conclusion
Three patients (4%) ceased thiopurine therapy due to thiopurine-related AEs, while no laboratory toxicity required therapy adjustments. Monitoring frequency of every 6 months seems feasible in patients with stable IBD on long-term (median duration > 6 years) maintenance thiopurine therapy and may contribute to reduced patient-burden and health care costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thiopurines, including azathioprine (AZA), mercaptopurine (MP), and thioguanine (TG), are immunosuppressive therapies for maintaining steroid-free remission in inflammatory bowel disease (IBD) [1,2,3]. The safety profile of thiopurines requires clinical monitoring because in up to 40% of thiopurine-treated patients with IBD, drug-related adverse events (AEs) lead to discontinuation of therapy [3,4,5,6,7,8,9,10,11,12,13,14]. Dose-independent AEs usually manifest within 6 months after the start of thiopurines and include flu-like syndrome, gastrointestinal intolerance (nausea), arthralgia, and pancreatitis [3, 4, 6,7,8,9,10,11, 13, 15,16,17,18,19]. Dose-dependent AEs can develop during long-term use and/or occur unexpectedly, and include hepatotoxicity, myelotoxicity, and infections [3, 4, 6,7,8,9,10,11, 13, 15,16,17,18,19,20]. Frequent clinical monitoring is advised for timely detection and management of drug-related AEs [5, 8, 9, 15, 21, 22].
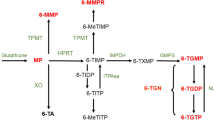
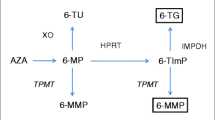
Thiopurines are metabolized to the active metabolite 6-thioguanine nucleotide (6-TGN) and supratherapeutic 6-TGN levels can induce myelotoxicity [23, 24]. Thiopurines are also converted into the methylated metabolite 6-MMP at the cost of 6-TGN and elevated 6-methylmercaptopurine (6-MMP) levels are associated with hepatotoxicity [25, 26]. Current guidelines from the American Gastroenterological Association (AGA) Institute, European Crohn’s and Colitis Organization (ECCO), and British Society of Gastroenterology (BSG) recommend regular laboratory assessment every 3 to 4 months to assess safety of thiopurine therapy [22, 27, 28]. These recommendations do not take into account the total years of thiopurine exposure, while severe AEs are rare after long-term thiopurine use [9, 15, 16, 29]. A recent retrospective cohort study assessed the incidence rates and clinical consequences of laboratory toxicity in 1132 patients with IBD after 1 year of consecutive thiopurine treatment with a median follow-up of 3.3 years (until therapy cessation or end of study follow-up) [19]. Only 83 patients (7%) required therapy adjustments based on laboratory findings [19]. Thus, less frequent clinical monitoring, including laboratory assessment, might be feasible in long-term thiopurine users [19, 29]. Moreover, frequent outpatient visits and laboratory assessments place a high burden on patients with IBD and the health care budget, especially in this COVID era [30]. Studies about the safety of a reduced monitoring strategy in patients with IBD with long-term thiopurine therapy are lacking.
This study aimed to evaluate the feasibility, safety, and costs of a reduced monitoring strategy in thiopurine-treated patients with IBD in long-term steroid-free remission.
Materials and Methods
Study Design
This single-center prospective cohort study assessed the safety of a reduced monitoring strategy in thiopurine-treated patients with IBD in stable remission. This study was conducted at the Radboud University Medical Center in Nijmegen, The Netherlands between September 2017 and December 2018.
Patient visits at the outpatient IBD clinics were scheduled at baseline and 12 months with an additional appointment by phone at six months with a specialized IBD nurse. In addition, laboratory assessment was routinely performed at 6 and 12 months. During follow-up, patients were advised to contact their physician or IBD nurse in case of suspected disease activity or AEs.
Patient Population
Patients ≥ 18 years of age with an established diagnosis of Crohn’s disease (CD), ulcerative colitis (UC), or IBD-unclassified (IBD-U) who were treated with weight-dosed thiopurine monotherapy (including AZA, MP, TG) for more than 6 months were eligible. Patients had to be in corticosteroid-free remission during ≥ 6 months prior to baseline as defined below. Long-term steroid-use was only allowed for treatment of comorbidities.
Exclusion criteria were concomitant treatment with biologic agents or prior use of biologic agents or corticosteroids up to six months preceding baseline, disease activity (biochemical and/or clinical), or ongoing AEs related to thiopurines. Allowed co-medication included long-term use and stable doses (≥ 6 months) of allopurinol and mesalamine.
Data Collection
Baseline
At baseline, we collected demographics, medical history, disease phenotype according to the Montreal Classification, and current and prior medication use. Detailed information about previous and current thiopurine exposure (period of use and dose) and recent metabolite level (determined by the method of Lennard and Singleton, as previously published), defined as 2 months prior to or after baseline date, was collected [23, 31]. At baseline, patients underwent metabolite measurement.
Follow-up
The laboratory assessment at baseline, 6 and 12 months included complete blood count, liver chemistry including alanine transaminase (ALT) and alkaline phosphatase (AP), C-reactive protein (CRP), fecal calprotectin (FCP), and thiopurine metabolite levels at baseline and as needed during further follow-up. All reported infections were collected during follow-up. Furthermore, physician global assessment (PGA) and medication adjustments were registered. During the first year of follow-up, additional contacts were recorded separately in a prospective fashion. During the second year of follow-up, only the primary outcome (as defined below) was recorded.
Data to calculate health care use were collected 1 year prior to baseline and during 1 year of follow-up after intervention, and included contact moments with attending physicians and IBD nurses, IBD-related laboratory assessment, and extra IBD-related diagnostics (imaging techniques).
Outcomes and Definitions
The primary outcome was the incidence of thiopurine-related AEs that required adjustments in thiopurine treatment during 24 months follow-up, including thiopurine discontinuation and dose adjustment. Secondary outcomes were only available for the first 12 months of the study and included all thiopurine-related AEs including laboratory toxicity, IBD-related AEs, therapy adjustments due to disease flares, and costs concerning IBD-related health care 1 year prior to and during the first year of the reduced monitoring frequency of 6 months. AEs were defined as any medical occurrence during the study follow-up, unrelated or related to underlying IBD, medical treatment, or strategy. Serious AEs included serious, life-threatening AEs resulting in death, irreversible illness or hospital admission. Severity of AEs were graded according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) [32]. These AEs were categorized in thiopurine- and IBD-related AEs based on the discretion of the treating physician. Clinical remission was defined as steroid-free inactive disease without the need for step-up treatment with biologic agents based on the PGA in addition to biochemical remission as FCP ≤ 250 µg/g and/or CRP ≤ 5 mg/L. A disease flare was defined as disease activity that resulted in an adjustment of thiopurine therapy or initiation of a new drug to induce remission, as previously published [33].
Laboratory results were assessed for toxicity as previously published [19]. Laboratory toxicity was defined as myelotoxicity, including leukopenia or thrombocytopenia, and/or hepatotoxicity. Leukopenia was classified as mild (3.0–4.0 × 109/L), moderate (2.0–3.0 × 109/L), or severe (< 2.0 × 109/L) [18, 19]. Thrombocytopenia was classified as mild (100–150 × 109/L), moderate (50–100 × 109/L), or severe (< 50 × 109/L) [19]. Hepatotoxicity was defined as any abnormal liver tests above upper limit of normal (ULN), including an elevated AP (> 125 U/L) and/or ALT (> 45 U/L). Severity of hepatotoxicity was graded according to the CTCAE classification [32]. Grade 1 was defined as liver tests between normal ULN and 2.5 × ULN (AP 125.1–312.5 U/L, ALT 45.1–112.5 U/L), grade 2 was between 2.5 and 5.0 × ULN (AP 312.6–625 U/L, ALT 112.6–225 U/L), and grade 3 was between 5.0 and 20.0 × ULN (AP 625.1–2500 U/L, ALT 226–900 U/L) [19]. The window of therapeutic metabolite level of 6-TGN was defined as 235–450 pmol/8 × 108 red blood cells (RBC) and desired 6-MMP levels as equal or lower than 5700 pmol/8 × 108 RBC.
Statistical Methods
Normally distributed values were presented by mean and standard deviation (SD) and non-normally distributed values by median with interquartile range (IQR). Categorical variables were presented as proportions and compared using Pearson χ2 or Fisher’s exact test. The Kaplan–Meier curve was used to describe the time to thiopurine-related AE that required a therapy adjustment during the study period of 24 months and patients were censored when lost to follow-up or the thiopurine therapy was ceased. Health care costs were calculated by the total amount of health care consumption per patient times the actual prices [34, 35]. To compare the costs prior to and during the reduction of monitoring frequency a one-sample t test with bootstrapping (n = 10,000) was used. A p value < 0.05 was considered statistically significant. SPSS Statistics (IBM, version 26.0) was used for statistical analyses.
Results
Baseline Characteristics
We enrolled 85 patients with IBD (see Fig. 1). The median disease duration was 12.5 years (IQR 6.4–22.9). The majority of the patients were female (62%) and were diagnosed with CD (61%). In total, 6 patients (7%) had previous malignancies including non-melanoma skin cancer (n = 4), breast cancer (n = 1), and 1 patient with both diagnoses. Overall, 25 patients (29%) were previously exposed to anti-tumor necrosis factor (TNF) and the median thiopurine exposure prior to baseline was 6.7 years (IQR 3.8–10.9). At baseline, 47 patients (55.3%) were treated with AZA (median dose 125 mg/day, 0.9 mg/kg adjusted for allopurinol), 25 (29.4%) with MP (median dose 50 mg/day, 0.8 mg/kg adjusted for allopurinol), and 13 (15.3%) with TG (median dose 20 mg/day). One patient used prednisolone for rheumatoid arthritis (5 mg/day). Baseline characteristics and thiopurine therapies are shown in Tables 1 and 2.
Thiopurine Therapy Interventions
In total, 3 patients (3.5%) ceased thiopurines and none underwent dose adjustments of thiopurine therapy due to thiopurine-related AEs. The reasons for thiopurine cessation (Table 3) included previous multiple infections and an ongoing infection (n = 1, thiopurine ceased 2 weeks after inclusion), gastrointestinal complaints (n = 1, thiopurine ceased 15 months after inclusion), and manifestation of non-melanoma skin cancer (n = 1, thiopurine ceased 17 months after inclusion, Fig. 2). Median months of follow-up was 23.9 (IQR 21.8–24.8).
Survival curve of thiopurine-related adverse events. Survival curve of time to thiopurine-related adverse events that required therapy adjustment during 24 months of follow-up following a reduced monitoring strategy. Number at risk table includes patients with loss to follow-up and all reasons that resulted in thiopurine cessation
Thiopurine-Related AEs Including Laboratory Abnormalities
During follow-up, 69 patients (81.2%) reported 122 AEs (Table 4). Overall, 25 thiopurine-related AEs were reported and included infections (n = 11), gastrointestinal complaints (n = 3), cutaneous lesions (n = 3), arthralgia (n = 1), fatigue (n = 1), and other reasons (n = 6), all not resulting in adjustments of the thiopurine therapy. No additional therapies were started due to thiopurine-related AEs. In 2 patients, extra laboratory assessment was performed, and in 4 patients extra outpatient visits were performed. None of the thiopurine-related AEs resulted in hospitalization.
During the first 12 months of follow-up, we observed 25 laboratory toxicities in 20 patients (Table 5). None resulted in thiopurine therapy adjustments. Overall, 9 patients (10.6%) experienced a mild leukopenia and 2 patients (2.4%) thrombocytopenia (1 mild, 1 moderate). We observed 14 events of laboratory hepatotoxicity (16.5%) including 4 mildly elevated AP, 9 mildly, and 1 moderately elevated ALT. All laboratory toxicity were transient and resolved without therapy adjustments.
Non-safety-related Adjustments in Thiopurine Therapy
There were several alternative reasons (other than AE-related) to cease or adjust the thiopurine therapy. High 6-TGN- and/or 6-MMP-metabolite levels were the reason of thiopurine dose adjustment in 6 patients (7.1%) and thiopurine therapy cessation in 2 patients (2.4%) at baseline. Other reasons for thiopurine cessation (n = 34, 40%) during 24 months of follow-up were stable remission (n = 26) and patient preferences (n = 8). Median time to thiopurine cessation was 22.0 months (IQR 18.6–24.0) for patients in stable remission, and 23.0 months (IQR 20.2–24.0) for patients stopping for personal preference. During follow-up, in 14 cases of dose adjustments were performed due to abnormal metabolite levels (n = 5, including n = 2 supratherapeutic and n = 3 subtherapeutic 6-TGN levels), remission (n = 4), patient preferences (n = 4), and failed tapering (n = 1). Two patients were lost during follow-up of 24 months. Dose adjustments during follow-up are shown in Table 3. In total, 46 patients (54.1%) continued thiopurine therapy throughout 24 months of follow-up.
Additional AEs and Health Care Benefit
Of the IBD-related AEs (n = 38 patients), most were gastrointestinal complaints (n = 20), arthralgia (n = 10), fatigue (n = 4), anemia (n = 2), cutaneous lesions (n = 1), and episcleritis (n = 1) (Table 4). New therapies were introduced in 29 patients due to disease flares including budesonide (n = 19), beclomethasone/mesalamine enema (n = 14), 5-ASA (n = 12), prednisolone (n = 5), allopurinol (n = 3), infliximab (n = 3), adalimumab (n = 2), golimumab (n = 1), and vedolizumab (n = 1). Of the 122 AEs, 12.3% were unrelated to either thiopurine therapy or IBD.
From the health care perspective there was a net benefit of €136.13 (p value 0.069, confidence interval, CI − 5.44 to 281.34) of reducing the monitoring frequency concerning all IBD-related contact moments with attending physicians and nurses, laboratory assessment, and diagnostics. Reasons for hospitalization during the first 12 months of follow-up (n = 3) were pneumonia with low 6-TGN levels and thiopurine continuation (n = 1), urogenital lesion (Hunner’s lesion) (n = 1), and anxiety disorder (n = 1) (Table 4).
Thiopurine Metabolites
Measurement of baseline 6-TGN metabolite levels was performed in 74 patients (87.1%) and showed therapeutic levels of 6-TGN in 30 patients (35.3%), subtherapeutic levels of 6-TGN (of which 3 patients < 50 pmol/8 × 108 RBC) in 22 patients (25.9%), and supratherapeutic levels of 6-TGN in 22 patients (25.9%). Measurement of baseline 6-MMP metabolite levels was performed in 62 patients (72.9%) and was within the normal range in 59 patients (69.4%) and was high in 3 patients (3.5%). The thiopurine use characteristics are shown in Table 2.
Leukopenia occurred in patients with baseline 6-TGN levels that were subtherapeutic (n = 1), therapeutic (n = 5), and supratherapeutic (n = 2), thrombocytopenia with subtherapeutic (n = 1) and therapeutic levels (n = 1). Elevated ALT (> 45 U/L) (n = 6) occurred with normal 6-MMP levels (n = 5) and with high 6-MMP levels (n = 1), while the 6-MMP levels were normal in all cases (n = 3) with elevated AP (> 125 U/L).
Discussion
We prospectively monitored a carefully selected group of long-term (median duration > 6 years) thiopurine-treated patients with IBD after implementation of a 6-monthly clinical monitoring strategy. In total, 3.5% ceased thiopurine therapy due to thiopurine-related AEs during 24 months of follow-up, while none of the laboratory toxicity we found resulted in thiopurine dose adjustments. Thiopurine treatment was continued in 54.1% until 24 months and the main reason for thiopurine cessation was stable remission (30.6%), whereas abnormal metabolite levels were the main reason for thiopurine dose adjustments (12.9%). None of the thiopurine-related patient reported AEs required hospitalization.
We found a low rate (3.5%) of thiopurine-related AEs that led to thiopurine therapy adjustments during 24 months of follow-up. Although multiple studies have described thiopurine-related AEs and monitoring strategies during the first year of use, limited evidence is available for patients with long-term thiopurine use. The incidence rates of thiopurine-related laboratory toxicity leading to therapy adjustments were assessed during a 3-monthly monitoring strategy in a high-volume multicenter retrospective cohort [19]. Overall, 6% ceased thiopurine therapy due to AEs versus 3.5% in our study [19]. The AEs reported in the latter study comprised 2.6% clinical AEs (including general malaise, skin reactions, arthralgia, and other reasons) and laboratory toxicity in 3.5% [19]. One important reason to explain these differences between studies is that the median thiopurine use at the time of inclusion in the previous study was shorter (1.1 years) compared to our cohort (6.7 years) [19]. The laboratory toxicity, AEs, and discontinuation rates are higher in the early phases of thiopurine use and may explain the differences between the two cohorts [3, 4, 6,7,8,9,10,11, 13, 15,16,17,18,19]. Furthermore, metabolite level-driven dose adjustments were performed in our study and this could have resulted in less AEs.
In our study, incidence rates of myelotoxicity and hepatotoxicity were 12.9% and 16.5%, respectively, but none resulted in therapy adjustments. In previous studies, aberrant laboratory results, independent of therapeutic consequences, had a broad range (0.5–32.7% myelosuppression versus 3.2–24.3% hepatotoxicity) [3,4,5,6,7,8,9,10, 12, 13, 15, 16, 18, 19, 21, 22, 29, 36]. More importantly, the observed laboratory toxicity in our study were transient, not severe, and did not have therapeutic consequences. This is in line with the literature showing that laboratory toxicity requiring therapy adjustment after long-term thiopurine use remains low (0.6–1.4% leucopenia, 2.1–3.2% hepatotoxicity) [3, 16, 19, 29].
In the current study, enrolled patients with stable remission of their IBD on long-term thiopurine therapy developed AEs but only few required thiopurine therapy adjustments or discontinuation. It is well known that AEs often occur in the first 6 to 12 months after thiopurine initiation [3,4,5,6,7,8,9,10,11,12,13,14]. Importantly, a previous cohort study observed lower incidence rates of infection, myelotoxicity, hepatotoxicity, pancreatitis, and neoplasms in patients with IBD using thiopurines for more than 4 years compared to patients with a median exposure of only 1 year since thiopurine initiation [13]. Moreover, thiopurine discontinuation predominantly occurred within the first 3 months of therapy [10]. In our study, the 3 thiopurine-related AEs that resulted in treatment cessation occurred after a median 6.7 years of thiopurine use.
An important question is whether we missed AEs due to a reduced frequency of monitoring. Previous cohort studies suggest that most cases of severe leukopenia occurred abruptly (within 1 month) [7]. We still observed a diverse number of AEs and laboratory toxicity that did not result in therapy adjustments, emphasizing that following a reduced monitoring strategy still results in a sufficient detection of AEs. Most of the AEs did not result in therapy adjustments and carry less clinical relevance, and this is in line with the previous studies [8, 10, 15, 29]. Second, AEs that needed therapy adjustment included clinical symptoms and probably would not have been detected with laboratory monitoring. Third, therapy adjustment based on monitoring of 6-TGN and 6-MMP levels, as applied in our study, may have reduced the risk of laboratory toxicity.
We observed a net benefit of 136 euro per patient for IBD-related health care use when scheduling outpatient visits and laboratory assessment every 6 months instead of every 3 to 4 months. We did not include medication costs, travel costs, work disability, or absenteeism. Including these items would likely increase the net benefit even more but a full cost-effectiveness analysis was beyond the scope of this study.
The strengths of this study include the careful selection of our study population with stable remission and long duration of thiopurine use prior to enrollment. Secondly, the prospective design and the extensive collection of AEs during 12 months is an addition to previously reported research of mostly retrospective nature. Our study also comes with several limitations. The main limitation is the lack of a control group monitored at the conventional 3-monthly strategy. To overcome this limitation, we compared our results with other cohorts [10, 19], however, previous cohorts and selected outcomes differed in methodology including selection criteria and design, not allowing for a comprehensive comparison. Second, the reduction of monitoring frequency resulted in fewer assessments and thereby a possible reduction in the detection rate of AEs. This could have resulted in an underestimation of the true AE incidence rates. It is likely that the strategy of reduced monitoring resulted in a more on-demand-based clinical health care as indicated by the additional non-scheduled contacts with physicians by patients. Third, the relatively low dosing of AZA (0.8 mg/kg, in part due to combination therapy with allopurinol) and the total drop-out (n = 39) after 24 months of follow-up reduced the generalizability of this study, although most patients (66.7%, n = 26) discontinued thiopurine because of stable remission rather than disease activity or AEs. The long median thiopurine exposure of 6.7 years should be taken into account as well. This suggests that gastroenterologists could consider thiopurine discontinuation in a group of patients with quiescent IBD tolerating long-term thiopurine therapy, but it is unclear whether these results can be extrapolated to patients with shorter duration of thiopurine use.
Overall, AEs that resulted in thiopurine therapy interventions were rare and the detected laboratory toxicity were transient and did not require therapy adjustments. In accordance with the current literature, most AEs were observed within the first 6 to 12 months after start of thiopurines [3,4,5,6,7,8,9,10,11,12,13,14].
Conclusion
Our study suggests that a monitoring frequency of every 6 months is safe and might be feasible for patients with IBD in stable remission on long-term thiopurine therapy beyond the induction phase. The reduction of monitoring frequency in this selected group of patients with IBD may contribute to the reduction of clinical visits and health-related costs.
Abbreviations
- AE(s):
-
Adverse event(s)
- AGA:
-
American Gastroenterological Association Institute
- ALT:
-
Alanine transaminase
- AP:
-
Alkaline phosphatase
- AZA:
-
Azathioprine
- BSG:
-
British Society of Gastroenterology
- CD:
-
Crohn’s disease
- CI:
-
Confidence interval
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CRP:
-
C-reactive protein
- FCP:
-
Fecal calprotectin
- IBD:
-
Inflammatory bowel disease
- IBD-U:
-
IBD-unclassified
- IQR:
-
Interquartile range
- MP:
-
Mercaptopurine
- PGA:
-
Physician Global Assessment
- RA:
-
Rheumatoid arthritis
- RBC:
-
Red blood cells
- SD:
-
Standard deviation
- TG:
-
Thioguanine
- UC:
-
Ulcerative colitis
- ULN:
-
Upper limit of normal
- 6-TGN:
-
6-Thioguanine nucleotide
- 6-MMP:
-
6-Methylmercaptopurine
References
Harbord M, Eliakim R, Bettenworth D et al. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J Crohns Colitis 2017;11:769–784.
Gionchetti P, Dignass A, Danese S et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis 2017;11:135–149.
Stournaras E, Qian W, Pappas A, et al. Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in Crohn's disease: long-term outcomes for 11,928 patients in the UK inflammatory bowel disease bioresource. Gut 2020.
Moran GW, Dubeau MF, Kaplan GG et al. Clinical predictors of thiopurine-related adverse events in Crohn’s disease. World J Gastroenterol 2015;21:7795–7804.
Goldberg R, Irving PM. Toxicity and response to thiopurines in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol 2015;9:891–900.
Chaparro M, Ordas I, Cabre E et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 2013;19:1404–1410.
Gisbert JP, Gomollon F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol 2008;103:1783–1800.
Gisbert JP, Gonzalez-Lama Y, Mate J. Thiopurine-induced liver injury in patients with inflammatory bowel disease: a systematic review. Am J Gastroenterol 2007;102:1518–1527.
Wallace TM, Veldhuyzen van Zanten SJ. Frequency of use and standards of care for the use of azathioprine and 6-mercaptopurine in the treatment of inflammatory bowel disease: a systematic review of the literature and a survey of Canadian gastroenterologists. Can J Gastroenterol 2001;15:21–28.
Jharap B, Seinen ML, de Boer NK et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis 2010;16:1541–1549.
Teml A, Schaeffeler E, Herrlinger KR et al. Thiopurine treatment in inflammatory bowel disease: clinical pharmacology and implication of pharmacogenetically guided dosing. Clin Pharmacokinet 2007;46:187–208.
Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut 2002;50:485–489.
Saibeni S, Virgilio T, D’Incà R et al. The use of thiopurines for the treatment of inflammatory bowel diseases in clinical practice. Dig Liver Dis 2008;40:814–820.
Broekman M, Coenen MJH, van Marrewijk CJ et al. More Dose-dependent Side Effects with Mercaptopurine over Azathioprine in IBD Treatment Due to Relatively Higher Dosing. Inflamm Bowel Dis 2017;23:1873–1881.
Bermejo F, Aguas M, Chaparro M et al. Recommendations of the Spanish Working Group on Crohn’s Disease and Ulcerative Colitis (GETECCU) on the use of thiopurines in inflammatory bowel disease. Gastroenterol Hepatol 2018;41:205–221.
Present DH, Meltzer SJ, Krumholz MP et al. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med 1989;111:641–649.
Warman JI, Korelitz BI, Fleisher MR et al. Cumulative experience with short- and long-term toxicity to 6-mercaptopurine in the treatment of Crohn’s disease and ulcerative colitis. J Clin Gastroenterol 2003;37:220–225.
Broekman M, Coenen MJH, Wanten GJ et al. Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment Pharmacol Ther 2017;46:953–963.
Kreijne JE, de Vries AC, de Veer RC, et al. Limited added value of laboratory monitoring in thiopurine maintenance monotherapy in inflammatory bowel disease patients. Aliment Pharmacol Ther 2020.
Dassopoulos T, Sultan S, Falck-Ytter YT et al. American Gastroenterological Association Institute technical review on the use of thiopurines, methotrexate, and anti-TNF-α biologic drugs for the induction and maintenance of remission in inflammatory Crohn’s disease. Gastroenterology 2013;145:1464–78.e1-5.
Connell WR, Kamm MA, Ritchie JK et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut 1993;34:1081–1085.
Lamb CA, Kennedy NA, Raine T et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106.
Wong DR, Coenen MJ, Vermeulen SH et al. Early Assessment of Thiopurine Metabolites Identifies Patients at Risk of Thiopurine-induced Leukopenia in Inflammatory Bowel Disease. J Crohns Colitis 2017;11:175–184.
de Boer NKH, Peyrin-Biroulet L, Jharap B et al. Thiopurines in Inflammatory Bowel Disease: New Findings and Perspectives. J Crohns Colitis 2018;12:610–620.
van Gennep S, Konté K, Meijer B et al. Systematic review with meta-analysis: risk factors for thiopurine-induced leukopenia in IBD. Aliment Pharmacol Ther 2019;50:484–506.
Meijer B, Kreijne JE, van Moorsel SAW et al. 6-Methylmercaptopurine-induced leukocytopenia during thiopurine therapy in inflammatory bowel disease patients. J Gastroenterol Hepatol 2017;32:1183–1190.
Maaser C, Sturm A, Vavricka SR et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis 2019;13:144–164.
Feuerstein JD, Nguyen GC, Kupfer SS et al. American Gastroenterological Association Institute Guideline on Therapeutic Drug Monitoring in Inflammatory Bowel Disease. Gastroenterology 2017;153:827–834.
Lewis JD, Abramson O, Pascua M, et al. Timing of myelosuppression during thiopurine therapy for inflammatory bowel disease: implications for monitoring recommendations. Clin Gastroenterol Hepatol 2009;7:1195–201; quiz 1141–2.
Te Groen M, Derks MEW, Kuijpers C et al. Reduction in Inflammatory Bowel Disease Healthcare During the Coronavirus Disease 2019 Pandemic: A Nationwide Retrospective Cohort Study. Gastroenterology 2021;160:935-937.e1.
Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr 1992;583:83–90.
Trotti A, Colevas AD, Setser A et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181.
de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet 2017;390:959–968.
Hakkaart-van Roijen L VdLN, Bouwmans C, et al. Kostenhandleiding. Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg.: Zorginstituut Nederland. 2015.
CZ. Tarieven Medisch Specialistische Zorg per 1 januari 2022, 2022.
Sluiter RL, Van Marrewijk C, De Jong D et al. Genotype-Guided Thiopurine Dosing Does not Lead to Additional Costs in Patients With Inflammatory Bowel Disease. J Crohns Colitis 2019;13:838–845.
Acknowledgments
Guarantors of the article Fenna M. Jansen, MD, Pepijn W.A. Thomas, MD, Nathan den Broeder, Frank Hoentjen, MD, PhD.
Funding
No funding has been received for this study.
Author information
Authors and Affiliations
Contributions
Study concept and design: LS, NB, and FH. Data acquisition: FJ, LS, DJ, and FH. Data analysis and interpretation: FJ, PT, NB, and FH. Drafting the manuscript: FJ, PT, NB, and FH. Critical revision of the manuscript for important intellectual additions: FJ, LS, PT, NB, DJ, JK, WD, and FH. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
D.J. de Jong received consulting fees from Synthon, Pharma, Abbvie, and MSD, and travel fees from Falk Pharma, Takeda, Abbvie, MSD, Ferring, Vifor Pharma, and Cablon Medical. F. Hoentjen has served on advisory boards, or as speaker or consultant for Abbvie, Celgene, Janssen-Cilag, MSD, Takeda, Teva, Sandoz, and Dr. Falk, and has received unrestricted grants from Dr. Falk, Janssen-Cilag, Abbvie, Takeda. Other authors have no potential conflict of interest to disclose.
Ethical approval
This study was approved by the Radboud University Medical Center Committee on Research Involving Human Subjects (2017-3656).
Informed consent
All participants provided written consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conference Presentation
This study was presented digitally at the European Crohn’s and Colitis Organisation 2021 and at the United European Gastroenterology Week 2021.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jansen, F.M., Smits, L.J.T., Thomas, P.W.A. et al. Feasibility of Reduced Clinical Monitoring in Patients with Inflammatory Bowel Disease Treated with Thiopurine Therapy. Dig Dis Sci 68, 2936–2945 (2023). https://doi.org/10.1007/s10620-023-07950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07950-0