Abstract
Background and Aims
Cirrhotic patients presenting with spontaneous bacterial peritonitis (SBP) have elevated risk of short-term mortality. While high Model for End-Stage Liver Disease-Sodium score (MELD-Na) and ascites culture yielding multi-drug resistance (MDR) bacteria are well established risk factors for further aggravating mortality, the impact of individual, causative microorganisms and their respective pathogenesis have not been previously investigated.
Methods
This is a retrospective study of 267 cirrhotic patients at two tertiary care hospitals undergoing paracentesis from January 2015 to January 2021 who presented with ascitic PMN count > 250 cells/mm3. The primary outcome was SBP progression defined as death or liver transplantation within 1-month of paracentesis stratified by microorganism type.
Results
Of 267 patients with SBP, the ascitic culture yielded causative microorganism in 88 cases [median age 57 years (IQR 52–64)]; 68% male; median MELD-Na 29 (IQR 23–35). The microbes isolated were E. coli (33%), Streptococcus (15%), Klebsiella (13%), Enterococcus (13%), Staphylococcus (9%) and others (18%); 41% were MDR. Cumulative incidence of SBP progression within 1-month was 91% (95% CI 67–100) for Klebsiella, 59% (95% CI 42–76) for E. coli, and 16% (95% CI 4–51) for Streptococcus. After adjusting for MELD-Na and MDR, risk of SBP progression remained elevated for Klebsiella (HR 2.07; 95% CI 0.98–4.24; p-value = 0.06) and decreased for Streptococcus (HR 0.28; 95% CI 0.06–1.21; p-value = 0.09) compared to all other bacteria.
Conclusion
Our study found Klebsiella-associated SBP had worse clinical outcomes while Streptococcus-associated SBP had the most favorable outcomes after accounting for MDR and MELD-Na. Thus, identification of the causative microorganism is crucial not only for optimizing the treatment but for prognostication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
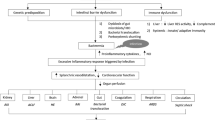
Spontaneous bacterial peritonitis (SBP) is one of the most devastating complications of cirrhosis and is associated with an extremely high mortality rate ranging from 20 to 40% [1]. SBP is an infection of ascitic fluid and occurs frequently in patients with advanced cirrhosis [1]. The pathophysiology underlying SBP is considered multifactorial, primarily mediated through the impaired intestinal immunity in decompensated cirrhosis along with overgrowth of gut microbiota, which predisposes to bacterial translocation into mesenteric lymphatics and subsequently to the peritoneum [2]. An insufficiency of peritoneal antibacterial immunity is considered a secondary prerequisite as it permits colonization of translocated bacteria towards the development of SBP following systemic dissemination of the pathogen.
The delay in SBP diagnosis and treatment initiation is coupled with increasing risk of death [3]. Thus, prompt diagnosis and administration of appropriate antibiotics is the critical determinant for successful management, which requires the identification of the causative pathogen. Obligate anaerobic bacteria outnumber aerobic bacteria by more than 100-fold in gut microbiota, however they rarely translocate from the gastrointestinal (GI) tract to the ascitic fluid [4]. Conversely, specific bacteria types (such as gram-negative organisms) are more adept at translocating to mesenteric lymph nodes as compared to other bacteria types (such as gram-positive organisms) conceivably as a result of a greater ability to adhere to the intestinal mucosal surface [5]. Consequently, gram-negative organisms are more commonly isolated from the ascitic fluid of SBP cases; thereby forming the basis of guidelines recommending the empiric use of a third-generation cephalosporin as the first choice and this has traditionally been the standard of care for the last few decades [6]. This recommendation also applies for the treatment of culture-negative neutrocytic ascites (CNNA), as this entity is considered a SBP subtype except that the ascitic culture fails to yield the responsible pathogen.
On the other hand, the epidemiology of gut microbiota has been changing rapidly over the past few decades. The increasing rates of SBP cases with gram-positive and multi-drug resistant (MDR) organisms have been documented widely in studies from European countries as well as the U.S. [1, 7]. While these epidemiological changes presumably involve multifaceted mechanisms, it has been speculated that the widespread abuse of antibiotics worldwide is one of the predominant factors. Consequently, there has been an exponential increase in the number of SBP cases with bacterial pathogens that are resistant to the first-line therapy; thereby associated with worse outcomes including mortality even with prompt transition to 2nd-line therapies [1]. The evolution of MDR organisms also compromises the preventative management of SBP. Prophylactic antibiotic therapy has been shown to effectively reduce the incidence of SBP; however, SBP in individuals who are on prophylaxis have a higher risk of MDR microorganisms, which are associated with worse prognosis [8].
These observations collectively indicate that the characteristics of bacterial pathogens have a direct relevance to the clinical presentations including outcomes and therapeutic decision-making in patients presenting with SBP. While the clinical significance of MDR bacteria has been well established, there has been sparse information on the impact of bacterial taxonomy on clinical presentations and outcomes. To date, there has not been a study investigating the pathogenesis of individual organisms in patients with SBP. Consequently, the purpose of this study is to elucidate the epidemiology and microbiology of bacterial pathogens isolated from the ascitic fluid of SBP cases and their correlation with clinical presentation and outcomes, which can be exploitable to improve the outcomes of patients with SBP.
Materials and Methods
Study Design and Patient Populations
This is a retrospective study conducted at two large academic tertiary care centers in Los Angeles, California. The study was approved by the Institutional Review Board at both LAC + USC Medical Center and Keck Hospital of USC.
The study included adults (≥ 18 years) hospitalized at either hospital from 01/2015 to 01/2021 for SBP in patients with cirrhosis. Cirrhosis was determined based on clinical, laboratory, histopathology, or radiographic data. SBP was defined as ascitic absolute neutrophil [often referred to as polymorphonuclear (PMN)] count ≥ 250 cells/mm3 without evidence of intra-abdominal, surgically treatable source of infection on either diagnostic or therapeutic paracentesis [9]. Culture-positive SBP is defined as the growth of one species of bacteria or fungus in ascitic fluid within five days of culture set-up [10]. All patients received standard of care, empiric antibiotics upon presentation.
Cases with suspected peritoneal carcinomatosis, hepatocellular carcinoma outside of the Milan criteria, polymicrobial growths (≥ 2 organisms), or peritoneal dialysis were excluded given their higher likelihood of secondary peritonitis. In addition, patients with cirrhosis due to autoimmune hepatitis who were on immunosuppressive therapies, or previous liver transplant (LT) cases were excluded. When repeat episodes of SBP were noted, we captured the last episode since patients may have had SBP at outside hospitals and been referred our tertiary center for subsequent episodes.
Data Collection
Data were collected retrospectively from 01/2015 to 01/2021, ascertaining patient demographics, etiology of cirrhosis, MELD-Na score, ascitic PMN counts, albumin at diagnosis of SBP, types of causative pathogens, MDR status, SBP prophylaxis use, mortality, release to hospice or liver transplant (LT). MDR is defined as resistance to at least three antimicrobial drug classes, extensively drug resistant if only sensitive to one or two classes and pandrug-resistant if resistant to all classes [11].
Outcomes
The primary outcome was SBP progression, the composite event of mortality or LT. Mortality was defined as death from any cause or release to hospice because these patients presumably die in hospice and their deaths are not readily reported to hospitals. LT is included as a primary endpoint as it is the only curative treatment for decompensated liver failure in patients with high MELD-Na scores who would have died if they did not receive a LT.
Statistical Analysis
Patient characteristics are presented as frequencies (percentages) and medians (interquartile ranges (IQR)) by bacterial pathogen. Differences by outcome were assessed using χ2 and Wilcoxon rank sum tests, respectively, applying exact tests when needed.
The Kaplan–Meier method was used to estimate the cumulative incidence of SBP progression at 1- and 6 months after the diagnostic paracentesis by the type of causative bacteria. Event rates were compared using the log-rank test with Sidak adjusted p-values to account for multiple comparisons. Patients were followed from the time of the diagnostic paracentesis to SBP progression or date of last follow-up with the hospital system (censored). Patients were censored at last follow-up to avoid assuming that those without 1- or 6 months follow-up did not experience the event. Cox regression estimated hazard ratios (HR) and 95% confidence intervals (CI) for risk of SBP progression within 1-month by bacterial type. We chose the 1-month outcome as most events could be attributed to SBP and for comparison to prior studies [10]. Bacterial pathogens were modeled as Klebsiella, Streptococcus, or other based on the Kaplan–Meier curves demonstrating similar event rates for the remaining bacteria. MDR and MELD-Na were selected a priori for inclusion in the multivariable model because they are well established predictors for mortality in SBP patients. Multicollinearity was assessed with variance inflation factors less than 10 confirmed. Analysis was completed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
267 cirrhosis patients whose PMN count in ascitic fluid exceeded 250 cells/mm3 were identified, of which ascitic fluid cultures yielded a causative pathogen in 88 patients as follows: E. coli (33%), Streptococcus (15%), Klebsiella (13%), Enterococcus (13%), Staphylococcus(9%) and others (Pseudomonas, Enterobacter, Listeria, Pasteurella, Citrobacter, Lactobacillus, Serratia and fungal; 18%). Two patients had SBP due to fungal infections. The median age at paracentesis was 57 years (IQR 52–64); 68% male; 59% Hispanic (Table 1). Cirrhosis was predominantly caused by alcohol (61%) with other etiologies being hepatitis C (18%), non-alcoholic fatty liver disease (12%), hepatitis B (5%), primary biliary cholangitis (1%), and cryptogenic or unknown (3%). Patients with end-stage renal disease on hemodialysis comprised 23% of the study group. Only 30 (34%) patients had a prior episode of documented SBP with 27 (31%) patients on SBP prophylactic antibiotics at time of admission.
Upon presentation, baseline characteristics and laboratory findings were obtained (Table 1). Overall, median MELD-Na was 29 (IQR 23–35), lowest for Streptococcus at 28 (IQR 16–30) and highest for Enterococcus and Staphylococcus at 32 (IQR 25–36 and 23–35, respectively). White blood cell count, systemic inflammatory response syndrome (SIRS), concurrent bacteremia, and serum albumin were similar by bacteria type. The overall median lactate was 3.6 (IQR 2.2–7.2) with Klebsiella having the highest value at 13.9 (IQR 2.7–11.0). Median absolute PMN leukocyte count was 2723 cells/mm3 (IQR 866–6381) with Streptococcus having the highest at 5500 cells/mm3 (IQR 2275–8424). Overall, 64 patients (73%) met SIRS diagnosis criteria on admission, in which all cases with Klebsiella had SIRS (100%). At time of paracentesis, 14 (16%) of total patients were on vasopressors with only one case associated with Streptococcus (7.7%). MDR status was seen in 36 (41%) patients with E. coli having the highest frequency (76%) and Streptococcus the lowest (0%). Of the organisms with MDR, 18 (50%) were extensively drug resistant with Klebsiella having the highest XDR at 3 (75%) (Table 1). When assessing for ESBL in patients with MDR, E. coli had 20 (91%) and Klebsiella had 2 (50%). Furthermore, 5 (83%) of patients with MDR Enterococcus had Vancomycin-resistant Enterococcus. No patients had documented Carbapenem-resistant Enterobacteriaceae.
SBP progression occurred in 57 patients, 45 (79%) within 1 month including 10 having LT, and 35 died of which 3 were released to hospice (Table 2). The cumulative incidence of SBP progression was highest for Klebsiella and lowest for Streptococcus (p < 0.001) with no statistically significant differences detected for the remaining bacterial types (Fig. 1). At 1-month after paracentesis, the cumulative incidence of SBP progression for Klebsiella was 91% (95% CI 67–100), Staphylococcus 66% (95% CI 32–95), Enterococcus 64% (95% CI 35–91), other 64% (95% CI 4–87), and E. coli 59% (95% CI 42–76) while Streptococcus was 16% (95% CI 4–51). For comparison, 1-month cumulative incidence of SBP was 40% (95% CI 32–48) for culture-negative neutrocytic ascites (Supplemental Fig. 1).
Clinical characteristics are compared by SBP progression within 1-month (Table 3). The distribution of bacteria type differed significantly by outcome (p < 0.001); a higher percentage with Klebsiella had SBP progression (22%) versus no progression (2%) and lower percentage with Streptococcus (4% vs 26%, respectively). Median MELD-Na (33 vs 24, p < 0.001) and MDR (21% vs 15%, p = 0.19) were higher for those with SBP progression, although the latter did not achieve statistical significance. Patients with SBP progression also had higher median WBC (12.8 vs 8.4, p = 0.003), lactate (6.4 vs 2.4, p ≤ 0.001), frequency of SIRS (38% vs 26%, p = 0.016), and vasopressor requirements (13% vs 1%, p ≤ 0.001) at presentation.
When examining 1-month risk of SBP progression in univariable analysis, risk increased with the presence of Klebsiella (HR 2.68, 95% CI 1.31–5.49) and decreased with the presence of Streptococcus (HR 0.18, 95% CI 0.04–0.77) compared to all other bacteria (Table 4). Additionally, for every 1-unit increase in MELD-NA, the risk of SBP progression increased by 11% (95% CI 1.06–1.16) while MDR had a 61% increased risk of SBP progression (95% CI 0.89–2.89) but was not statistically significant. After adjusting for MELD-Na and MDR in a multivariable analysis, SBP progression remained elevated for Klebsiella with a twofold increased risk (HR 2.07, 95% CI 0.988–4.24) and decreased for Streptococcus with HR of 0.28 (95% CI 0.06–1.21) compared to all other bacteria.
Discussion
Overall, the development of SBP is associated with higher mortality and morbidity rates. In particular, SBP cases with renal dysfunction, high MELD scores, and hospital-acquired are associated with poor clinical outcomes. Apart from these host factors, our understanding of the role of bacterial virulence factors has been limited, with the exception of the link between MDR status of the causative pathogen and poor clinical outcomes [8]. Accordingly, little is known whether the bacterial pathogen correlates with clinical outcomes as compared to other identifiable bacteria. Our study revealed that, for the first time, SBP cases with Klebsiella pneumoniae are associated with worse outcomes such as high mortality, transfer to hospice or liver transplantation, as demonstrated by a hazard ratio twice that compared to other identifiable bacteria. This is further supported when comparing SBP progression for Klebsiella compared to culture-negative neutrocytic ascites (Supplemental Fig. 1). Moreover, our study showed that SBP with Klebsiella pneumoniae, regardless of MDR status, is associated with the highest WBC levels, lactate levels and highest percentage of meeting SIRS criteria. Conversely, cases with Streptococcus have the most favorable outcomes, even after adjusting for MDR and MELD-Na. These observations collectively suggest that the type of bacteria, along with sensitivity to empiric therapy with 3rd generation cephalosporin, might serve as a critical and independent determinant for clinical outcomes of SBP.
Classically, Klebsiella pneumoniae has been considered a commensal and opportunistic microorganism; therefore, the progression to overt infection requires additional risk factors, which include but are not limited to diabetes, alcohol use, fluid, and electrolyte disorders [12], all of which are highly relevant to patients with advanced cirrhosis, the patients represented in our study. Historically, Klebsiella pneumoniae has accounted for a relatively small proportion SBP cases compared with other pathogens such as E. coli, consisting of approximately 10% of all SBP cases depending on the region and ethnicity [13]. However, the number of SBP cases with Klebsiella pneumoniae is expected to grow as epidemiological studies demonstrated an increasing prevalence of colonization over the last decades both at the community level as well as in hospitalized patients. Currently, the rate of community acquired Klebsiella pneumoniae colonization in nasopharyngeal and GI tract are as high as 15% and 35%, respectively. Of note, the rate of GI tract colonization in hospitalized patients, especially those who had been exposed to antibiotics, has been reported to be nearly 80% [14,15,16]. Since the GI tract is the predominant reservoir site for the SBP pathogens, the endemic colonization strongly suggests a spike in the number of cases with Klebsiella pneumoniae [17].
The increasing Klebsiella prevalence is concerning; however, this alone does not fully explain the previously unrecognized high pathogenicity of SBP with Klebsiella pneumoniae, for which the evolution of virulence factors likely serves as an explanation according to cumulative evidence. Recent epidemiological studies with molecular and genetic approaches demonstrated the emergence of strains harboring genes responsible for enhanced virulence [18, 19]. The acquisition of virulence factors as well as antibiotics resistant genes are largely mediated through genetic alterations of the accessory genome [20,21,22,23,24,25,26]. For example, the well-accepted hyper-virulence factors, RmpA, K2A, and MagA genes are all encoded in the accessory genome [27, 28]. Due to the high flexibility of the accessory genome for the horizontal gene transfer among species, the emergence of dual-risk strains that carry both antibiotic-resistant and hypervirulent genes is becoming evident, which makes it progressively pathogenic and difficult to treat. This concept is not only applicable for SBP pathogenesis but also for infections in other organ systems. Indeed, over the past 30 years, the increasing incidence of liver abscesses and septic metastasis caused by hypervirulent strains of Klebsiella pneumoniae has been reported, and has emerged as a leading cause of pyogenic liver abscesses, initially documented in several Asian countries and now in the United States [29].
Detection of virulence factors requires the genotyping of the accessory genome which is beyond the capacity of a typical clinical microbiology laboratories [30]. Accordingly, this is one of the limitations of the present study, as we are unable to report on virulence factors and thus, cannot definitively correlate poor outcomes in cases with Klebsiella pneumoniae results with the presence of bacterial virulence. An assessment of hypervirulent Klebsiella pneumoniae carriage rates at the local community, regional, and national levels is required to define the generalizability of our study results. Another limitation is the relatively small number of patients for each identified organism group. However this study contained one of the largest number of culture-positive SBP subjects in the United States. Taken together, further prospective molecular epidemiological studies with a larger number of subjects along with molecular genetic approaches are warranted.
Conclusion
Our study is the first to demonstrate the significance of bacteria type on clinical outcomes after adjusting for known risk factors (MDR and MELD-Na) in cirrhotic patients presenting with SBP. Currently, ascitic fluid cultures are performed mainly to determine MDR status by performing sensitivities; however, our study highlights the importance of determining bacterial types for management strategy and prognostication. Lastly, this study is the first to report worse clinical outcomes with Klebsiella and favorable clinical outcomes with Streptococcus after adjusting for MDR and MELD-Na.
Abbreviations
- GI:
-
Gastrointestinal
- MELD-Na:
-
Model for End-Stage Liver Disease-Sodium
- LT:
-
Liver transplant
- MDR:
-
Multidrug-resistant
- PMN:
-
Polymorphonuclear leukocyte
- SBP:
-
Spontaneous bacterial peritonitis
- TIPS:
-
Transjugular intrahepatic portosystemic shunt
- XDR:
-
Extensively drug resistant
References
Ariza X, Castellote J, Lora-Tamayo J et al. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol 2012;56(4):825–832.
Alexopoulou A, Apadopoulos N, Eliopoulos DG et al. Increasing frequency of gram-positive cocci and gram-negative multidrug-resistant bacteria in spontaneous bacterial peritonitis. Liver Int 2013;33(7):975–981.
Kim JJ, Tsukamoto MM, Mathur AK et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol 2014;109(9):1436–1442.
Steffen E, Berg R, Deitch E. Comparison of translocation rates of various indigenous bacteria from the gastrointestinal tract to the mesenteric lymph node. J Infect Dis 1988;157:1032–1038.
Ljungdahl M, Lundholm M, Katouli M et al. Bacterial translocation in experimental shock is dependent on the strains in the intestinal flora. Scand J Gastroenterol 2000;35(4):389–397.
Dupeyron C, Campillo B, Mangeney N et al. Changes in nature and antibiotic resistance of bacteria causing peritonitis in cirrhotic patients over a 20 year period. J Clin Pathol 1998;51(8):614–616.
Fernandez J, Prado V, Trebicka J et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J Hepatol 2019;70(3):398–411.
Fernandez J, Acevedo J, Castro M et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012;55(5):1551–1561.
Hoefs JC, Canawati HN, Sapico FL et al. Spontaneous bacterial peritonitis. Hepatology 1982;2(4):399–407.
Sunjaya DB, Lennon RJ, Shah VH et al. Prevalence and predictors of third-generation cephalosporin resistance in the empirical treatment of spontaneous bacterial peritonitis. Mayo Clin Proc 2019;94(8):1499–1508.
Alexopoulou A, Vasilieva L, Agiasotelli D et al. Extensively drug-resistant bacteria are an independent predictive factor of mortality in 130 patients with spontaneous bacterial peritonitis or spontaneous bacteremia. World J Gastroenterol 2016;22(15):4049–4056.
Meatherall BL, Gregson D, Ross T et al. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med 2009;122(9):866–873.
Dever JB, Sheikh MH. Review article: Spontaneous bacterial peritonitis–bacteriology, diagnosis, treatment, risk factors and prevention. Aliment Pharmacol Ther 2015;41(11):1116–1131.
Davis TJ, Matsen JM. Prevalence and characteristics of Klebsiella species: relation to association with a hospital environment. J Infect Dis 1974;130(4):402–405.
Dao TT, Liebenthal D, Tran TK et al. Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS ONE 2014;9:e91999.
Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 1998;11(4):589–603.
Dorman MJ, Short FL. Genome watch: Klebsiella pneumoniae: when a colonizer turns bad. Nat Rev Microbiol 2017;15:384.
Palacios M, Miner TA, Frederick DR et al. Identification of two regulators of virulence that are conserved in Klebsiella pneumoniae classical and hypervirulent strains. mBio. 2018. https://doi.org/10.1128/mBio.01443-18.
Shon AS, Russo TA. Hypervirulent Klebsiella pneumoniae: the next superbug? Future Microbiol 2012;7(6):669–671.
Bialek-Davenet S, Criscuolo A, Ailloud F et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis 2014;20(11):1812–1820.
Holt KE, Wertheim H, Zadoks RN et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci USA 2015;112(27):E3574-3581.
Brisse S, Verhoef J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, GYRA and PARC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol 2001;51(Pt 3):915–924.
Brisse S, van Himbergen T, Kusters K et al. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin Microbiol Infect 2004;10(10):942–945.
Brisse S, Passet V, Grimont PA. Description of Klebsiella quasipneumoniae sp. Nov., isolated from human infections, with two subspecies, Klebsiella quasipneumoniae subsp. Quasipneumoniae subsp. Nov. And Klebsiella quasipneumoniae subsp. Similipneumoniae subsp. Nov., and demonstration that Klebsiella singaporensis is a junior heterotypic synonym of Klebsiella variicola. Int J Syst Evol Microbiol 2014;64:3146–3152.
Maatallah M, Vading M, Kabir MH et al. Klebsiella variicola is a frequent cause of bloodstream infection in the stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS ONE 2014;9:e113539.
Berry GJ, Loeffelholz MJ, Williams-Bouyer N. An investigation into laboratory misidentification of a bloodstream Klebsiella variicola infection. J Clin Microbiol 2015;53(8):2793–2794.
Liu C, Du P, Xiao N et al. Hypervirulent Klebsiella pneumoniae is emerging as an increasingly prevalent K. pneumoniae pathotype responsible for nosocomial and healthcare-associated infections in Beijing, China. Virulence 2020;11(1):1215–1224.
Cubero M, Marti S, Dominguez MA et al. Hypervirulent Klebsiella pneumoniae serotype k1 clinical isolates form robust biofilms at the air-liquid interface. PLoS ONE 2019;14:e0222628.
Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 2005;100(2):322–331.
Russo TA, Marr CM. Hypervirulent Klebsiella pneumonia. Clin Microbiol Rev. 2019. https://doi.org/10.1128/CMR.00001-19.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study was supported in part by the USC Research Center for Liver Disease (P30DK048522).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-023-07865-w.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Furey, C., Zhou, S., Park, J.H. et al. Impact of Bacteria Types on the Clinical Outcomes of Spontaneous Bacterial Peritonitis. Dig Dis Sci 68, 2140–2148 (2023). https://doi.org/10.1007/s10620-023-07867-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-023-07867-8