Abstract
Background and Aims
HIV-positive patients on tenofovir hydroxyl fumarate (TDF)/emtricitabine have a lower risk of COVID-19 and hospitalization than those given other treatments. Our aim was to analyze the severity of COVID-19 in patients with chronic hepatitis B (CHB) on TDF or entecavir (ETV).
Methods
Spanish hospital databases (n = 28) including information regarding adult CHB patients on TDF or ETV for the period February 1st to November 30th 2020 were searched for COVID-19, defined as a positive SARS-CoV-2 polymerase chain reaction, and for severe COVID-19.
Results
Of 4736 patients, 117 had COVID-19 (2.5%), 67 on TDF and 50 on ETV. Compared to patients on TDF, those on ETV showed (p < 0.05) greater rates of obesity, diabetes, ischemic cardiopathy, and hypertension. COVID-19 incidence was similar in both groups (2.3 vs. 2.6%). Compared to TDF, patients on ETV more often (p < 0.01) had severe COVID-19 (36 vs. 6%), required intensive care unit (ICU) (10% vs. 0) or ventilatory support (20 vs. 3%), were hospitalized for longer (10.8 ± 19 vs. 3.1 ± 7 days) or died (10 vs. 1.5%, p = 0.08). In an IPTW propensity score analysis adjusted for age, sex, obesity, comorbidities, and fibrosis stage, TDF was associated with a sixfold reduction in severe COVID-19 risk (adjusted-IPTW-OR 0.17, 95%CI 0.04–0.67, p = 0.01).
Conclusion
Compared to ETV, TDF seems to play a protective role in CHB patients with SARS-CoV-2 whereby the risk of severe COVID-19 is lowered.
Similar content being viewed by others
Introduction
Since December 2019, more than 307 million people have become infected by SARS-CoV-2 and at least 5 million deaths have been declared by COVID-19 [1]. Despite vaccination, most people remain vulnerable to COVID-19 because of heterogeneity in vaccine administration among countries and emergence of SARS-CoV-2 variants that escape immunization. These facts have fostered research on new antivirals or repurposing of older ones that could be effective against SARS-CoV-2.
Tenofovir disoproxil fumarate (TDF) is a nucleotide analog indicated in the treatment of patients with chronic hepatitis B (CHB) to suppress hepatitis B virus DNA levels [2] and combined with emtricitabine and/or lamivudine in patients with HIV [3]. Three observational studies in HIV-positive individuals conducted during the first pandemic wave found the prevalence of SARS-CoV-2 antibodies, and the risks of symptoms, hospitalization, and death by COVID-19 were lower among patients on antiviral regimens that include TDF [4,5,6]. The results of these studies have been confirmed in a large cohort of HIV-positive individuals after careful adjustment for multiple comorbidities [7]. Protection from SARS-CoV-2 infection or severe COVID-19 by TDF could also be of relevance in patients with CHB. Approximately, 240 million people are living with chronic HBV infection, and some of them are candidates to therapy with nucleot(s)ide analogs, TDF, or entecavir (ETV) [2]. In them, demonstration of a benefit of TDF on COVID-19 could matter in the decision making of CHB therapy.
Most of the published studies of COVID-19 in patients with CHB focus on the incidence and clinical characteristics. Rates of incidence ranging from 0.1 to 12.2% have been found, depending on country’s HBV prevalence. Previous liver dysfunction and also COVID-19-related liver injury seem to worsen prognosis [8,9,10,11,12,13]. Other studies analyze the possible reactivation of CHB during COVID-19 with different results [14, 15]. However, no study has evaluated the potential impact of the nucleotide analog used to treat CHB on COVID-19 outcomes.
We have evaluated in a Spanish nationwide cohort of patients with CHB on treatment with nucleot(s)ide analogs, TDF or ETV, the severity of COVID-19 during the first 10 months of pandemia.
Patients and Methods
Study Design and Patients
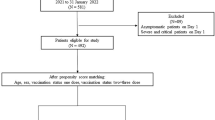
This industry-independent and observational retrospective study was conducted according to STROBE statement. The databases of 28 Spanish hospitals of patients with CHB on TDF or ETV were searched for cases of COVID-19 between 1st February and 30th November 2020. All databases were cross-checked with in-hospital pharmacy registries to ensure the inclusion of all CHB patients on antiviral treatment.
Inclusion criteria were (i) age above 18 years; (ii) CHB defined as HBsAg positive for at least six months; (iii) antiviral treatment with TDF or ETV at the time of COVID-19 diagnosis; and (iv) SARS-CoV-2 infection defined by a positive PCR result of nasopharyngeal swab test done both in primary and hospital care. Patients with more than one antiviral drug or coinfected with HIV, HCV, or HDV were excluded.
For each confirmed case, age, sex, comorbidities as obesity, diabetes mellitus, arterial hypertension, and ischemic cardiopathy were registered. COVID-19 clinical features, management, hospital stay, and outcomes were collected by medical history. Liver fibrosis was assessed from the most recent liver elastography or liver biopsy available.
Data were anonymized and collected from medical records by each local investigator and were centrally compiled and analyzed. The study was approved by all ethics committees of the participating institutions. Individual written informed consent was waived by ethic committees due to the study design and purposes.
Definitions
Severe COVID-19 was defined according to the World Health Organization criteria: severe pneumonia (respiratory rate > 30 breaths/min, or oxygen saturation < 94% on room air), acute respiratory distress syndrome (PaO2/FiO2 < 300), sepsis, or septic shock [16]. COVID-19 clinical severity was classified as outpatient, hospital admission, or intensive care unit (ICU) admission [16]. Ventilatory support was defined as non-invasive mechanical ventilation or orotracheal intubation requirements. Length of hospitalization was calculated in days from the date of hospital admission to the date of discharge or death.
Statistical Analysis
Qualitative variables are expressed as frequency counts and percentages. Quantitative variables are expressed as the median and 1st–3rd quartile. Proportions were compared using the χ2 or Fisher’s exact test, and continuous variable were tested using parametric (t test) and non-parametric test (Mann–Whitney U test) when appropriate. To calculate the propensity score of receiving TDF, we fitted a logistic model including age, sex, obesity, arterial hypertension, diabetes mellitus, ischemic cardiopathy, HBeAg presence, detectable HBV DNA, and advanced fibrosis [17]. Then, we used inverse probability of treatment weighting (IPTW) propensity score method to compare COVID-19 severity between patients receiving TDF or ETV. We assessed the balance of covariates by an overidentification test and by calculating raw and weighted standardized differences. Need for intensive care unit (ICU) and ventilatory support, and mortality were explored by bivariate analysis due to the low number of events. Significance was set at p < 0.05. Data were analyzed using the SPSS Statistics package version 22.0 (SPSS Inc, Chicago, IL).
Results
Baseline Characteristics of COVID-19 Cases
The databases of the participating hospitals included 4736 patients with CHB, 1864 of on ETV (39%) and 2872 on TDF (61%). The search identified 117 cases of COVID-19 (8-month incidence = 2.5%, 95%CI 2.1–2.9%) (Supplementary Table 1). Seventeen patients (14.5%) were obese, and 24 (20.5%) were previously diagnosed with diabetes mellitus, 34 (29.1%) with arterial hypertension, and 9 (7.7%) with ischemic cardiopathy. Fifty out of the 117 patients (42.7%) were on ETV and 67 on TDF (57.3%), and the median treatment duration was of 60 (24.5–107) months. Twenty-one patients were HBeAg positive, and 22 had detectable HBV DNA. Twenty-eight (23.9%) patients presented advanced fibrosis (F3 or F4). COVID-19 hospitalization was needed in 46 patients (39.3%), 22 (18.8%) presented severe COVID-19, and 5 of them required ICU admission (4.3%). Twelve patients (10.3%) received ventilatory support and 6 (5.1%) died.
Comparisons Between Groups of Treatment
Compared with patients on TDF (Table 1), those on ETV were older, and had significantly (p < 0.05) greater rates of obesity, diabetes, ischemic cardiopathy, and arterial hypertension. There were no significant differences between groups in HBeAg status, detectable HBV DNA, or treatment duration. Advanced fibrosis was non-significantly more frequent in patients on ETV (32 vs. 18%, p = 0.06).
The incidence of COVID-19 in patients on TDF or ETV was similar (0.023 vs. 0.026, p = 0.44). ETV patients more often (p < 0.01) had severe COVID-19 (36% vs. 6%), required ICU (10% vs. 0), ventilatory support (20% vs. 3%), had longer hospitalization (10.8 ± 19 vs. 3.1 ± 7 days), or died (10% vs. 1.5%, p = 0.08).
Severe COVID-19 Analysis
Univariate analysis indicates that severe COVID-19 was significantly (p < 0.05) more frequent in patients who were older [odds ratio (OR) age > 60 years 3.79 (95%CI 1.43–10)], had diabetes mellitus [OR 3.69 (95%CI 1.34–10.16)], or were on ETV [OR 8.85 (95%CI 2.76–28.3)] (Table 2). Inverse probability of treatment weighting propensity score showed that TDF treatment reduced by sixfold the risk of severe COVID-19 (adjusted OR 0.17, 95%CI 0.04–0.68, p = 0.013). Supplementary Table 2 and the overidentification test (p = 0.97, the null hypothesis that the covariates were balanced cannot be rejected) show that covariates were well-balanced after IPTW matching.
Discussion
In this study, we have addressed the incidence and severity of COVID-19 in a large nationwide cohort of 4736 patients with CHB on ETV or TDF during the first pandemic wave. Our results show that after adjustment for multiple comorbidities and severity of chronic liver disease, TDF treatment exerts a protective effect for severe COVID-19 infection when compared with ETV.
Molecular studies have set the experimental basis for a beneficial impact of TDF on COVID-19. The coronaviruses are single-strand RNA viruses whose replication requires a RNA-dependent RNA polymerase. Both HIV and HBV incorporate TDF in their polymerases, which stops viral replication [18]. Molecular docking and in vitro studies indicate that TDF inhibits SARS-CoV-2 RNA polymerase [19,20,21]. Moreover, TDF/emtricitabine reduces viral loads in nasal swabs of preclinical models [22]. TDF also has immunomodulatory effects, including decreased production of interleukin-8 and -10 [23], both have been shown to predict COVID-19 severity. Interestingly, tenofovir alafenamide (TAF) lacks activity against SARS-CoV-2 in a cell model, has weaker immunomodulatory effects, and reaches tenfold lower extracellular concentrations and mucosal penetrations than TDF, which justify that HIV individuals on TAF fared no better than the general population from COVID-19 [4].
Our results indicate that TDF exerts protective effects in CHB patients with COVID-19. This was shown by the lower ICU admission, ventilatory support need, hospitalization length, and deaths in patients on TDF compared to those on ETV. This conclusion has been reached after adjustment for relevant covariates, including comorbidities, by means of the IPTW method. These results agree with those studies that have shown lower COVID-19 severity in HIV patients on antiviral regimens that include TDF [4,5,6,7]. In contrast, we did not observe that TDF reduced COVID-19 incidence in patients with CHB compared to those on ETV. Conclusions regarding incidence in retrospective observational studies are rather inaccurate and biased by differences in health provision behavior. Specifically, studies of TDF in HIV patients were limited to the first months of the pandemic when PCR testing was restricted to hospitals and mild COVID-19 cases recommended not to come to hospitals, having lower access to PCR testing. In contrast, our study extended observations through 2020, an interval when mild COVID-19 was diagnosed in hospitals and in outpatient clinics, a factor that could have attenuated the differences between treatment groups.
The strengths of our study include its national scope, relatively long-time interval, and analysis and adjustment for a variety of potential risk factors and comorbidities. However, several limitations should be acknowledged. The study is retrospective and we cannot discard some bias in patient selection and residual confounding. We also should consider that limitation of PCR testing to hospitals in the first months of the pandemic could have contributed to underestimate mild COVID-19 cases. Conclusions regarding mortality in our cohort are limited by the short number of deaths. Finally, our results involve alfa SARS-CoV 2 variant, so our findings deserve further investigations with newer variants like delta or omicron.
As the SARS-CoV-2 pandemic keeps going, a protective effect of TDF from severe COVID-19 in patients with CHB could be of interest in certain situations in which TDF might be considered the preferred treatment option of CHB: (i) patients with advanced liver disease in whom vaccines are likely to be suboptimal, (ii) patients with recent CHB diagnosis who are about to initiate antivirals, since it is unlikely to switch long-term treatments, and (iii) immunosuppressed patients who need antivirals to prevent HBV reactivation.
In conclusion, the results of this nationwide study indicate that compared to ETV, TDF exerts a protective effect in CHB patients infected with SARS-CoV-2, with less risk of severe COVID-19.
References
COVID-19 Map. Johns Hopkins Coronavirus Resource Center. [cited 2021 Mar 11]. https://coronavirus.jhu.edu/map.html
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398.
Arribas JR, Polo R, García JG, Palacios R. Esteban Martínez (GeSIDA). 145.
del Amo J, Polo R, Moreno S et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy. Ann Intern Med. 2020;173:536–541.
Hoffmann C, Casado JL, Härter G et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22:372–378.
Berenguer J, Díez C, Martín-Vicente M et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:1678–1684.
Rial-Crestelo D, Bisbal O, Font R, et al. Incidence and severity of SARS-CoV-2 infection in HIV-infected individuals during the first year of the pandemic. J Acquir Immune Defic Syndr. 2021
Wu J, Yu J, Shi X et al. Epidemiological and clinical characteristics of 70 cases of coronavirus disease and concomitant hepatitis B virus infection: a multicentre descriptive study. J Viral Hepat. 2021;28:80–88.
Lv X-H, Yang J-L, Deng K. COVID-19 patients with hepatitis B virus infection. Off J Am Coll Gastroenterol. 2021;116:1357–1358.
Alqahtani SA, Buti M. COVID-19 and hepatitis B infection. Antivir Ther. 2021.
Chen L, Huang S, Yang J et al. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27:1504–1507.
Ampuero J, Sánchez Y, García-Lozano MR et al. Impact of liver injury on the severity of COVID-19: a systematic review with meta-analysis. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig. 2021;113:125–135.
Guerra Veloz MF, Cordero Ruiz P, Ríos-Villegas MJ et al. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Espanola Enfermedades Dig Organo Of Soc Espanola Patol Dig. 2021;113:103–109.
Liu J, Wang T, Cai Q, et al. Longitudinal changes of liver function and hepatitis B reactivation in COVID‐19 patients with pre‐existing chronic HBV infection. Hepatol Res. 2020
Rodríguez-Tajes S, Miralpeix A, Costa J et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89–94.
Therapeutics and COVID-19: living guideline. [cited 2022 Jan 18]. https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2022.1
Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the Propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol. 2012;12:70.
Birkus G, Hájek M, Kramata P, Votruba I, Holý A, Otová B. Tenofovir diphosphate is a poor substrate and a weak inhibitor of rat DNA polymerases alpha, delta, and epsilon*. Antimicrob Agents Chemother. 2002;46:1610–1613.
Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020;15:117592.
Chien M, Anderson TK, Jockusch S, et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase. bioRxiv. 2020; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7239050/
Jockusch S, Tao C, Li X et al. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antiviral Res. 2020;180:104857.
Xie X, Muruato AE, Zhang X et al. A nanoluciferase SARS-CoV-2 for rapid neutralization testing and screening of anti-infective drugs for COVID-19. Nat Commun. 2020;11:5214.
Melchjorsen J, Risør MW, Søgaard OS et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57:265–275.
Acknowledgments
None.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. No funding sources needed.
Author information
Authors and Affiliations
Contributions
Conceptualization and methodology: AA, BMM, and SM. Data curation: BMM, MB, IF, MHC, VBM, FDF, RMM, LGB, EB, MM, AAN, SRT, LRM, AM, MGR, JIA, JC, JMGS, CFR, PC, MD, AM, AP, MR, EH, JJM, JT, MAS, CMF, JLC, RB, SL, JGS, JC, MRG, and FG. Formal analysis: BM, ERS, and AA. Writing original draft: BMM, AA, and SM. Writing, review and editing: BMM, MB, IF, MHC, VBM, FDF, RMM, LGB, EB, MM, AAN, SRT, LRM, AM, MGR, JIA, JC, JMGS, CFR, PC, MD, AM, AP, MR, EH, JJM, JT, MAS, CMF, JLC, RB, SL, JGS, JC, MRG, FG, ERS, SM, and AA..
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mateos-Muñoz, B., Buti, M., Vázquez, I.F. et al. Tenofovir Disoproxil Fumarate Reduces the Severity of COVID-19 in Patients with Chronic Hepatitis B. Dig Dis Sci 68, 2731–2737 (2023). https://doi.org/10.1007/s10620-022-07817-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07817-w