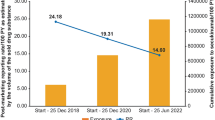
Abstract
Background and Aims
Children with Crohn’s disease have lower response rates to infliximab, lower infliximab levels, and higher infliximab clearance on weight-based dosing than adults. We hypothesize infliximab clearance is a predictive of later outcomes on infliximab in children with Crohn’s disease.
Methods
In this single-center retrospective study, data were collected from charts on diagnosis, anthropometry, routine labs, infliximab therapeutic drug monitoring, infliximab dosing, disease activity, and other treatments. With these data we generated a population pharmacokinetic model using non-linear mixed effects modeling and calculated infliximab clearance for each patient over time. Patients were classified as in remission, responder-only or non-responder at 5, 10 and 16 months. Regression and ROC analyses were used to assess for early predictors of remission and response to infliximab.
Results
Eighty-five subjects were included, with a median follow-up of 22.3 months (IQR 10.1–36.8). Our pharmacokinetic model showed infliximab clearance was positively associated with CRP and weight, while negatively associated with albumin. In regression analyses, early infliximab clearance was the only significant, consistent predictor of remission. A 0.1 L/day increase in infliximab clearance predicted remission with an OR between 0.179 and 0.426. Differences in dosing did not account for differences in outcome. Infliximab clearance alone had moderate predictive accuracy of remission, with an AUC between 0.682 and 0.738.
Conclusions
Early infliximab clearance is strongly associated with remission in children with Crohn’s disease. It may be useful as a marker of response in proactive therapeutic drug monitoring to guide early dose optimization and/or changes in treatment for betterment of long-term outcomes.



Similar content being viewed by others
References
Baldassano R, Braegger CP, Escher JC et al. Infliximab (REMICADE) therapy in the treatment of pediatric Crohn’s disease. Am J Gastroenterol. 2003;98:833–838.
Kelsen JR, Grossman AB, Pauly-Hubbard H et al. Infliximab therapy in pediatric patients 7 years of age and younger. J Pediatr Gastroenterol Nutr. 2014;59:758–762.
Fasanmade AA, Adedokun OJ, Blank M et al. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther. 2011;33:946–964.
Singh N, Dubinsky MC. Therapeutic drug monitoring in children and young adults with inflammatory bowel disease: a practical approach. Gastroenterol Hepatol (N Y). 2015;11:48–55.
Hemperly A, Vande CN. Clinical pharmacokinetics and pharmacodynamics of infliximab in the treatment of inflammatory bowel disease. Clin Pharmacokinet. 2018;57:929–942.
Dubinsky MC, Mendiolaza ML, Phan BL et al. Dashboard-driven accelerated infliximab induction dosing increases infliximab durability and reduces immunogenicity. Inflamm Bowel Dis. 2022;28:285.
Dubinsky MC, Phan BL, Singh N et al. Pharmacokinetic dashboard-recommended dosing is different than standard of care dosing in infliximab-treated pediatric IBD patients. AAPS J. 2017;19:215–222.
Dave MB, Dherai AJ, Desai DC et al. Optimization of infliximab therapy in inflammatory bowel disease using a dashboard approach-an Indian experience. Eur J Clin Pharmacol. 2021;77:55–62.
Bauman LE, Xiong Y, Mizuno T et al. Improved population pharmacokinetic model for predicting optimized infliximab exposure in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2020;26:429–439.
Frymoyer A, Hoekman DR, Piester TL et al. Application of population pharmacokinetic modeling for individualized infliximab dosing strategies in Crohn disease. J Pediatr Gastroenterol Nutr. 2017;65:639–645.
Papamichael K, Chachu KA, Vajravelu RK et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017;15:1580–1588.
Lyles JL, Mulgund AA, Bauman LE et al. Effect of a practice-wide anti-TNF proactive therapeutic drug monitoring program on outcomes in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2021;27:482–492.
Vaughn BP, Martinez-Vazquez M, Patwardhan VR et al. Proactive therapeutic concentration monitoring of infliximab may improve outcomes for patients with inflammatory bowel disease: results from a pilot observational study. Inflamm Bowel Dis. 2014;20:1996–2003.
Cohen RZ, Schoen BT, Kugathasan S et al. Management of anti-drug antibodies to biologic medications in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2019;69:551–556.
Unal I. Defining an optimal cut-point value in ROC analysis: an alternative approach. Comput Math Methods Med. 2017;2017:3762651.
Wu Q, Peters SA. A retrospective evaluation of allometry, population pharmacokinetics, and physiologically-based pharmacokinetics for pediatric dosing using clearance as a surrogate. CPT Pharmacometrics Syst Pharmacol. 2019;8:220–229.
Malik PRV, Edginton AN. Physiologically-based pharmacokinetic modeling vs. allometric scaling for the prediction of infliximab pharmacokinetics in pediatric patients. CPT Pharmacometrics Syst Pharmacol. 2019;8:835–844.
Petitcollin A, Leuret O, Tron C et al. Modeling immunization to infliximab in children with Crohn’s disease using population pharmacokinetics: a pilot study. Inflamm Bowel Dis. 2018;24:1745–1754.
Singh N, Rosenthal CJ, Melmed GY et al. Early infliximab trough levels are associated with persistent remission in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:1708–1713.
Zitomersky NL, Atkinson BJ, Fournier K et al. Antibodies to infliximab are associated with lower infliximab levels and increased likelihood of surgery in pediatric IBD. Inflamm Bowel Dis. 2015;21:307–314.
Colman RJ, Portocarrero-Castillo A, Chona D et al. Favorable outcomes and anti-TNF durability after addition of an immunomodulator for anti-drug antibodies in pediatric IBD patients. Inflamm Bowel Dis. 2021;27:507–515.
Grossi V, Lerer T, Griffiths A et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1748–1756.
Ungar B, Glidai Y, Yavzori M et al. Association between infliximab drug and antibody levels and therapy outcome in pediatric inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2018;67:507–512.
Cornillie F, Hanauer SB, Diamond RH et al. Postinduction serum infliximab trough level and decrease of C-reactive protein level are associated with durable sustained response to infliximab: a retrospective analysis of the ACCENT I trial. Gut. 2014;63:1721–1727.
Hofmekler T, Bertha M, McCracken C et al. Infliximab optimization based on therapeutic drug monitoring in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64:580–585.
Stein R, Lee D, Leonard MB et al. Serum infliximab, antidrug antibodies, and tumor necrosis factor predict sustained response in pediatric Crohn’s disease. Inflamm Bowel Dis. 2016;22:1370–1377.
Strik AS, Wang YC, Ruff LE et al. Individualized dosing of therapeutic monoclonal antibodies-a changing treatment paradigm? AAPS J. 2018;20:99.
Dotan I, Ron Y, Yanai H et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259.
Courbette O, Aupiais C, Viala J et al. Trough levels of infliximab at week 6 are predictive of remission at week 14 in pediatric Crohn’s disease. J Pediatr Gastroenterol Nutr. 2020;70:310–317.
Feuerstein JD, Nguyen GC, Kupfer SS et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834.
Mitrev N, Vande Casteele N, Seow CH et al. Review article: Consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053.
Vande Casteele N, Ferrante M, Van Assche G et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–1329.
Afonso J, Lopes S, Gonçalves R et al. Proactive therapeutic drug monitoring of infliximab: a comparative study of a new point-of-care quantitative test with two established ELISA assays. Aliment Pharmacol Ther. 2016;44:684–692.
Winter DA, Joosse ME, de Wildt SN et al. Pharmacokinetics, pharmacodynamics, and immunogenicity of infliximab in pediatric inflammatory bowel disease: a systematic review and revised dosing considerations. J Pediatr Gastroenterol Nutr. 2020;70:763–776.
Louis E, El Ghoul Z, Vermeire S et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment Pharmacol Ther. 2004;19:511–519.
Billiet T, Dreesen E, Cleynen I et al. A genetic variation in the neonatal Fc-receptor affects anti-TNF drug concentrations in inflammatory bowel disease. Am J Gastroenterol. 2016;111:1438–1445.
Zubin G, Peter L. Predicting endoscopic Crohn’s disease activity before and after induction therapy in children: a comprehensive assessment of PCDAI, CRP, and Fecal Calprotectin. Inflamm Bowel Dis. 2015;21:1386–1391.
Acknowledgments
We thank the nursing staff Cheryl Kluthe, Leanne Shirton and Jason Wang who were involved in the care of the patients in the study and assisted in some of the data collection. We are grateful for Women and Children's Health Research Institute (WCHRI) in providing resources such as Redcap support in this study. Lastly, we are grateful for the North American Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) Foundation’s supporting of the mentor-mentee relationship between Dr. Hien Huynh and Aaron Chung.
Funding
This research is funded by the generous support of the Stollery Children's hospital foundation through the Women and Children's Health Research Institute (WCHRI). Aaron Chung's summer studentship was supported by the 2021 North American Society For Pediatric Gastroenterology, Hepatology & Nutrition (NASPGHAN) Foundation’s Medical Student Mentored Summer Research Program.
Author information
Authors and Affiliations
Contributions
The following authors were involved in the conception and design of the study, and revising the article critically for important intellectual content: HQH, EW, MWC, DI. The following author was involved in the conception and design of the study, drafting the article, acquisition of data, and analysis and interpretation of data: AC. The following authors were involved in acquisition of data, analysis and interpretation of data, and revising the article critically for important intellectual content: DM, PA, AP. All authors were involved in final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
Hien Q Huynh has received educational grants from AbbVie and Janssen and served as an advisory board member for BioJamp. He has also received speaker fees from AbbVie, Janssen and Fresenius. Eytan Wine has received speaker fees from AbbVie, Janssen, Nestle Health Sciences, BioJamp, Pfizer, and Mead Johnson Nutrition. Matthew W Carroll has received speaker fees from AbbVie. Diane R Mould is the president of Projections Research Inc, a consulting company for the pharmaceutical industry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Conference Presentation
Name: 16th Congress of ECCO, ECCO-IBD 2021. Location: Virtual. Year: 2021. Work presented: A Chung, M Carroll, P Almeida, A Petrova, D Mould, E Wine, H Huynh, Edmonton Pediatric IBD Clinic (EPIC), P337 Early infliximab clearance predicts remission in children with Crohn’s Disease, Journal of Crohn's and Colitis, Volume 15, Issue Supplement_1, May 2021, Pages S362–S363, https://doi.org/10.1093/ecco-jcc/jjab076.461.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chung, A., Carroll, M., Almeida, P. et al. Early Infliximab Clearance Predicts Remission in Children with Crohn’s Disease. Dig Dis Sci 68, 1995–2005 (2023). https://doi.org/10.1007/s10620-022-07783-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07783-3