Abstract
Aim
Pancreatic fibrosis is the main pathological characteristic of chronic pancreatitis (CP) and pancreatic cancer. Pancreatic stellate cells (PSCs) play a critical role in pancreatic fibrosis. Any targets that may have an impact on the activation of PSCs could become potential treatment candidates for CP and pancreatic cancer. Our goal was to investigate the effect of P-element-induced wimpy-testis (PIWI) protein 1 (PIWIL1) on PSC activation.
Methods
Lentivirus-based RNA interference (RNAi) and overexpression vector construction were used to knock down and over-express the PIWIL1 protein. Immunocytofluorescent staining, western blotting, wound healing assay, transwell assay, and phalloidin staining were used to investigate the effects of PIWIL1 on the secretion of extracellular matrix components (EMC), actin cytoskeleton, and on the invasion and migration abilities of primary PSCs isolated from C57BL/6 mice. Moreover, pancreatic fibrosis was induced by l-arginine in C57BL/6 mice. The expression of PIWIL1 and collagen deposition in vivo were tested by western blotting and Sirius red staining.
Results
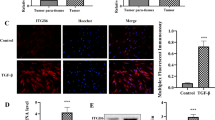
Expression levels of collagen I, collagen III, and α-smooth muscle actin were significantly decreased in the LV-PIWIL1 group. Compared with the si-PIWIL1 group, significant differences were observed in the expression of desmin, p-PI3K, p-AKT, and p-mTOR in the LV-PIWIL1 group. Furthermore, PIWIL1 suppressed the PSCs’ invasion and migration abilities. In a rescue experiment, the PI3K/AKT/mTOR signaling pathway was found to be the underlying mechanism in PSCs activation mediated by PIWIL1.
Conclusions
Our findings suggest that PIWIL1 inhibits the activation of PSCs via the PI3K/AKT/mTOR signaling pathway. PIWIL1 is a potential therapeutic target for pancreatic fibrosis.









Similar content being viewed by others
References
Xue R, Jia K, Wang J et al. A rising star in pancreatic diseases: pancreatic stellate cells. Front Physiol. 2018;9:754.
Xue R, Yang J, Jing W et al. Coenzyme Q10 inhibits the activation of pancreatic stellate cells through PI3K/AKT/mTOR signaling pathway. Oncotarget. 2017;8:92300–92311.
Zhang Z, Zhang H, Liu T et al. Heterogeneous pancreatic stellate cells are powerful contributors to the malignant progression of pancreatic cancer. Front Cell Dev Biol. 2021;9:783617.
Xue R, Wang J, Yang L et al. Coenzyme Q10 ameliorates pancreatic fibrosis via the ROS-triggered mTOR signaling pathway. Oxid Med Cell Longev. 2019;2019:8039694.
Zhang D, Zhao L, Luo M et al. Yap-Myc signaling induces pancreatic stellate cell activation through regulating glutaminolysis. Exp Cell Res. 2021;411:113000.
Manoukian P, Bijlsma M, van Laarhoven H. The cellular origins of cancer-associated fibroblasts and their opposing contributions to pancreatic cancer growth. Front Cell Dev Biol. 2021;9:743907.
Amrutkar M, Gladhaug IP. Stellate cells aid growth-permissive metabolic reprogramming and promote gemcitabine chemoresistance in pancreatic cancer. Cancers (Basel). 2021;13:601.
Yao F, Ma L. piRNA-unbound PIWIL1 promotes metastasis. Nat Cell Biol. 2020;22:359–360.
Li W, Martinez-Useros J, Garcia-Carbonero N et al. The prognosis value of PIWIL1 and PIWIL2 expression in pancreatic cancer. J Clin Med. 2019;8:1275.
Tan H, Zhu Y, Zheng X et al. PIWIL1 suppresses circadian rhythms through GSK3β-induced phosphorylation and degradation of CLOCK and BMAL1 in cancer cells. J Cell Mol Med. 2019;23:4689–4698.
Schnittert J, Bansal R, Prakash J. Targeting Pancreatic Stellate Cells in Cancer. Trends Cancer. 2019;5:128–142.
Xue R, Hua L, Wenbin X et al. Derivation and validation of the potential core genes in pancreatic cancer for tumor-stroma crosstalk. BioMed Research International. 2018;2018:4283673.
Pang TCY, Wilson JS, Apte MV. Pancreatic stellate cells: what’s new? Curr Opin Gastroenterol. 2017;33:366–373.
Pothula SP, Zhihong X, Goldstein D et al. Key role of pancreatic stellate cells in pancreatic cancer. Cancer Lett. 2016;381:194–200.
Ramakrishnan P, Loh WM, Gopinath SCB et al. Selective phytochemicals targeting pancreatic stellate cells as new anti-fibrotic agents for chronic pancreatitis and pancreatic cancer. Acta Pharm Sin B 2020;10:399–413.
Dong Peixin, Xiong Ying, Konno Yosuke et al. Critical roles of PIWIL1 in human tumors: expression, functions, mechanisms, and potential clinical implications. Front Cell Dev Biol. 2021;9:656993.
Yu Y, Xiao J, Hann SS. The emerging roles of PIWI-interacting RNA in human cancers. Cancer Manag Res. 2019;11:5895–5909.
Ozata DM, Gainetdinov I, Zoch A et al. PIWI-interacting RNAs: small RNAs with big functions. Nat Rev Genet. 2019;20:89–108.
Guo B, Li D, Likun D et al. piRNAs: biogenesis and their potential roles in cancer. Cancer Metastasis Rev. 2020;39:567–575.
Hempfling AL, Lim SL, Adelson DL et al. Expression patterns of HENMT1 and PIWIL1 in human testis: implications for transposon expression. Reproduction. 2017;154:363–374.
Ponnusamy M, Yan K-W, Liu C-Y et al. PIWI family emerging as a decisive factor of cell fate: An overview. Eur J Cell Biol. 2017;96:746–757.
Cheng Y, Wang Q, Jiang W et al. Emerging roles of piRNAs in cancer: challenges and prospects. Aging (Albany NY). 2019;11:9932–9946.
Liu J, Zhang S, Cheng B. Epigenetic roles of PIWI-interacting RNAs (piRNAs) in cancer metastasis (Review). Oncol Rep. 2018;40:2423–2434.
Shima G, Tehila A, Hamed A et al. Management and Treatment of Hepatocellular Carcinoma with Immunotherapy: A Review of Current and Future Options. J Clin Transl Hepatol. 2020;8(2):168–176.
Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2019.
Chang Y, Li H. J Hepatic antifibrotic pharmacotherapy: are we approaching success? J Clin Transl Hepatol. 2020;8:222–229.
Funding
The study was supported by the Fund for Fostering Young Scholars of Peking University Health Science Center (BMU2021PY010), National Natural Science Foundation of China (No. 82002461), and Medjaden Academy and Research Foundation for Young Scientists (MJR20211110).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, R., Zhou, J., Wu, J. et al. P-element-Induced Wimpy-Testis-Like Protein 1 Regulates the Activation of Pancreatic Stellate Cells Through the PI3K/AKT/mTOR Signaling Pathway. Dig Dis Sci 68, 1339–1350 (2023). https://doi.org/10.1007/s10620-022-07605-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07605-6