Abstract
Background
Cereals are known to trigger for wheat allergy, celiac disease and non-celiac wheat sensitivity (NCWS). Inflammatory processes and intestinal barrier impairment are suspected to be involved in NCWS, although the molecular triggers are unclear.
Aims
We were interested if different bread types influence inflammatory processes and intestinal barrier function in a mouse model of inflammatory bowel disease.
Methods
Epithelial caspase-8 gene knockout (Casp8ΔIEC) and control (Casp8fl) mice were randomized to eight groups, respectively. The groups received different diets for 28 days (gluten-free diet, gluten-rich diet 5 g%, or different types of bread at 50 g%). Breads varied regarding grain, milling and fermentation. All diets were isocaloric.
Results
Regardless of the diet, Casp8ΔIEC mice showed pronounced inflammation in colon compared to ileum, whereas Casp8fl mice were hardly inflamed. Casp8fl mice could tolerate all bread types. Especially yeast fermented rye and wheat bread from superfine flour but not pure gluten challenge increased colitis and mortality in Casp8ΔIEC mice. Hepatic expression of lipopolysaccharide-binding protein and colonic expression of tumor necrosis factor-α genes were inversely related to survival. The bread diets, but not the gluten-rich diet, also decreased colonic tight junction expression to variable degrees, without clear association to survival and inflammation.
Conclusions
Bread components, especially those from yeast-fermented breads from wheat and rye, increase colitis and mortality in Casp8ΔIEC mice highly susceptible to intestinal inflammation, whereas control mice can tolerate all types of bread without inflammation. Yet unidentified bread components other than gluten seem to play the major role.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat sensitivities increased extensively in the last years in industrialized western countries [1, 2]. Three types of wheat sensitivities can be distinguished, celiac disease (CD), wheat allergy (WA) and non-celiac wheat sensitivity (NCWS), with a prevalence of approximately 1% of adults each [3]. CD and WA are immunological diseases [4] while the origin of NCWS is unclear at present, but an involvement of the immune system and an impaired intestinal barrier are assumed to play a role [5, 6]. NCWS symptoms may also be secondary to low-grade type of chronic inflammation triggered by particular bread consumption [1, 7].
The prevalence of NCWS among patients with inflammatory bowel disease (IBD) seems to be much higher than in the normal population with a prevalence of up to 27.6% [8]. Individuals who report an IBD flare within the past 60 days were significantly more likely to report NCWS suggesting that this “wheat intolerance” or “bread intolerance” could be a transient phenomenon associated with an impaired intestinal barrier function that improves in IBD remission phases [8]. It has been reported that also CD might be related to IBD, since CD is a kind of risk factor for IBD [9] and microscopic colitis [10].
It has been shown that a proportion of patients with IBD benefit from a gluten-free diet [11], and IBD patients with NCWS reported higher disease activity as compared to IBD patients without NCWS [12]. The underlying triggering agents are not necessarily gluten-related, but could be also fermentable carbohydrates (FODMAPs) causing increased gas production [8]. Apart from triggers, manufacturing processes with a lower fermentation time and the use of highly efficient yeast species have been discussed as potentially relevant factors in the pathophysiology of NCWS, the first case of which was published in 1978 [13].
Milling, kneading, fermentation and heating impact the characteristics of bread and might also affect the composition of breads, especially the protein composition [14, 15].
Rizzello et al. [16] showed that specific sourdough microorganisms can lead to a decrease of allergenic proteins in wheat- and rye bread. Bread improvers in combination with thermal processing of the dough could lead to the formation of new allergens [15, 17].
This study aimed to evaluate the effects of different bread types and a gluten enriched diet in control and Casp8ΔIEC mice, a mouse model with intestinal inflammation. Loss of the caspase-8 gene has been shown to result in a loss of Paneth cells and a reduced expression of antimicrobial peptides, resulting in epithelial damage, an impaired gut barrier, and finally in a higher susceptibility to intestinal inflammation [18].
Methods
Bread Making
For preparing the different breads analyzed in our study we used grains from bread wheat (hereafter referred to as wheat), spelt and rye (Schapfenmühle, Ulm, Germany), from which superfine flour (SF) (Quadrumat junior mill, Brabender, Duisburg) and wholegrain flour (WG) (Ultra-centrifugal mill ZM 200 sieve insert 0.5 mm; Retsch, Haan) were produced. Details of bread manufacturing and results of flour analysis are described elsewhere [14]. For this study six different bread types were produced (Table 1). Bread improver (MeisterMarken Ulmer Spatz, type Weißback Super, CSM Deutschland GmbH, Bremen, Germany) was used for one bread type (W SF SB) and was composed of buttermilk powder (24%); wheat flour; soy flour; emulsifier (soya lecithins, mono- and diglycerides of fatty acids); wheat malt flour; dextrose; salt; acidity regulator (calcium acetate); sour whey powder; acidulant (citric acid, lactic acid); wheat swell flour; ascorbic acid; enzymes. Study breads differed regarding oligosaccharide content (Table S1).
Animals
In a preliminary test, we tested if healthy mice tolerate a diet with a high proportion of bread. Eight female Balb/c mice of an age of 11 weeks were fed for 28 days with a diet that consisted of 50 g% (50% of total food weight expressed in gram) of yeast-fermented wheat bread from superfine flower (W SF Y, see also Table 1). Food intake and weight gain were measured every day.
In the main part of our studies, mice with a specific deletion of caspase-8 in the intestinal epithelium (Casp8ΔIEC) bred on a C57BL/6 J genetic background and Cre-negative control littermates with floxed caspase-8 alleles (Casp8fl) were used. They were originally obtained from the laboratory of Christoph Becker (Medical Clinic, Erlangen, Germany) [18] and bred in our laboratory under specific pathogen-free (SPF) conditions. To avoid too serious spontaneous inflammation, 6-week-old female Casp8ΔIEC and Casp8fl mice as well as Cre-negative littermates were used. Mice genotypes were confirmed by DNA analysis from ear biopsies using polymerase chain reaction (PCR), specific for the respective allele. Before start of our experiments, all mice received ad libitum a gluten free breeding diet (S0514-E750, Ssniff Spezialdiäten GmbH, Soest, Germany). Both Casp8ΔIEC and Casp8fl were divided into eight groups with eight mice each. Each group was fed with a different diet offered in pellet form ad libitum for 28 days. The eight diets are listed in Table 2.
During the experiments mice were housed in a SPF barrier facility with a fully controlled environment at 22 ± 2 °C and 50% humidity, under a 12-h light/dark cycle accredited by the Association for Assessment and Accreditation for Laboratory Animal Care International. They were kept in collective cages containing two or three mice each. Our study was approved by the local Animal Care and Use Committee (Regional Council Stuttgart, V343/18 EM). All experiments were conducted according to the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA).
Dietary Treatment
Eight different custom diets (Ssniff Spezialdiäten GmbH) were produced for use in the experiments; (i) a gluten-free control diet based on rice (GF) and (ii) a gluten-free diet based on rice supplemented with 5 g% wheat gluten (Sigma-Aldrich, St. Louis, USA) (5G), which served as controls, and (iii–xiii) six different bread-diets, which consisted half/half of the corresponding bread (50 g%) and other nutrients except cereals (50 g%) to reach the nutritional requirements for mice. All experimental diets had the same total energy content of 14.6 MJ/kg (Table 2).
Human Equivalence Dose for Gluten
To assess the human equivalence dose (HED) for gluten consumption in our mouse experiments we used the formula HED = mouse dose (mg/kg) × (mouse Km/human Km), whereby Km is the ratio of body weight to body surface area for a given species (37 for humans, 3 for mice) [19]. In our experiments, mice received 125 mg/d of gluten in the 5G diet, or 20–70 mg/d of gluten in the bread diets. Considering an average mouse body weight of 20 g, this corresponds to 35.4 g/d of gluten (5G diet), or 5.6–19.8 g/d of gluten (bread diets) in a 70 kg man, respectively, which is in line with the average daily gluten ingestion in humans is estimated to be between 13 and 30 g/day [7, 20].
Feeding Period, End of the Experiment and Dropouts
Food intake and body weight of the mice were assessed three times a week during the feeding period of 4 weeks. The health status was checked daily by using internally established health scores to evaluate the health status. If mice met the criteria for premature study end like diarrhea or bloody feces, loss of body weight > 15%, or deteriorated general well-being, they were killed before the planned study end and defined as dropouts. Otherwise, the study terminated after 28 days, and mice were killed after blood had been collected from the portal vein. Mice were fasted for 4–6 h and then anesthetized with ketamine-xylazine (100:16 mg/kg body weight) by intraperitoneal injection 1 h after gavage with fluorescein isothiocyanate-dextran 4000 (FITC-D4000) (600 mg/kg body weight, using a stock volume of 50 mg/mL). Liver and gut tissue was collected and immediately frozen in dry ice or stored in neutral-buffered formalin.
Colonoscopy
To detect colitis and tissue necrosis in the course of the study, colonoscopy using a mouse video endoscopic system (Karl Storz Endoscope, Coloview system, Mainz, Germany) was performed as described [21].
Histological Analysis
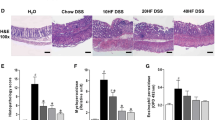
Colon and ileum samples were rinsed, collected and fixed in 10% neutral-buffered formalin (Sigma-Aldrich, St Louis, USA) for minimum 24 h and subsequently embedded in paraffin. Paraffin sections of 5 μm were cut and de-waxed prior to staining with hematoxylin/eosin (H&E; Merck, Darmstadt, Germany). Tissue sections were evaluated, and images taken by standard light microscopy using an AxioImager Z1 microscope (Carl Zeiss MicroImaging, Jena, Germany). Histological scoring occurred as already described [22]. Total histological score (sum of both the tissue damage score and the infiltration score of inflammatory cells) in ileum and colon tissue resulted in 0–6 points, which were interpreted as a low-grade histological score (LHSc, 0–2 points), a middle grade histological score (MHSc, > 2–4 points), and a high grade score (HHSc, > 4–6 points).
Small Intestinal Permeability
Blood from the portal vein was collected in heparinized 1.5 ml tubes for FITC-D4000 analyses as described elsewhere [23]. In short, fluorescence was measured in 96-well plates (Infinite M200 PRO, Tecan, Crailsheim, Germany) using a fluorimeter (Multi-Detection Microplate Reader, Synergy TM HT, Bio-Tek®, Vermont, USA) with an excitation wavelength of 485 nm and an emission wavelength of 528 nm. FITC-D4000 concentrations were calculated with the help of a standard curve of FITC-D4000 with concentrations ranging from 0 to 250 µg/ml.
Real-Time Quantitative Reverse Transcription-PCR
Total RNA was isolated from liver- and colon tissue using Trizol® reagent (Invitrogen Life Technologies, Carlsbad, USA) following the manufacturer’s instructions. Yield and purity of RNA was determined by NanoDrop ND-1000 spectrophotometer (NanoDrop 2000c, Thermo Fisher Scientific, Darmstadt, Germany). In addition, RNA integrity was checked using agarose gel electrophoresis. Intact RNA samples with an absorbance ratio OD 260/280 between 1.8 and 2.1 and OD 260/230 greater than 2.0 were used for further analysis. cDNA was synthesized from 1 μg of total RNA using the SuperScript® IV Reverse-Transcriptase (Thermo FisherScientific, Darmstadt, Germany) after DNAse treatment (Promega, Madison, USA). cDNA was stored at − 20 °C until use. Real-time PCR was conducted in Bio-Rad iQ5 Real- Time System. Eva Green Universal PCR Master Mix (Bio-Rad Laboratories, Munich, Germany) was used to prepare the PCR mix. The amplification program for primers: tumor necrosis factor-α (TNFα), mucin-2 (Muc2), zonula occludens-1 (ZO1), Occludin and Claudin-2 (CLDN2) was: 95 °C for 30 s, 40 cycles at 95 °C for 5 s and 60 °C for 30 s. For the primers of lipopolysaccharide-binding protein (LBP), toll-like receptor-4 (TLR4) and myeloid differentiation primary response-88 (MyD88) the program was: 95 °C for 30 s, 40 cycles at 95 °C for 5 s and 62 °C for 30 s. After amplification, a thermal denaturing cycle was added to derive the dissociation curve of the PCR product to verify amplification specificity. The comparative CT method was used to determine the amount of the target genes, normalized to an endogenous reference (β-actin mRNA expression) and relative to a calibrator (2−ΔΔCT). Primer sequences are given in Table 3.
Statistical Analysis
The results are shown as the means ± SEM if not indicated otherwise. Normal distribution was tested using Shapiro–Wilk test. To test the potential effects of the factors of diet (six bread diets, GF and 5G) and genotype (Casp8ΔIEC vs. Casp8fl) and their interaction, we performed two-way ANOVA with a Tukey’s multiple comparison test and inverse transformation of raw data in cases of unequal variance. Survival data were analyzed using the Kaplan–Meier method. Means and percentages of normally distributed variables were reported with their respective 95% confidence intervals (95% CI). A P-value < 0.05 was considered as statistically significant. For statistical analysis and figure presentations we used GraphPad Prism, version 9.2 (Graph Pad, La Jolla, CA) and SPSS, version 25 (IBM, Armonk, NY). For correlation analyses, we calculated Spearman’s correlation coefficients with SPSS, version 25 (IBM).
Results
Body Weight Change in Response to a Bread-Rich Diet in Control Mice
In a first set of experiments, we tested tolerance to a diet with a high bread portion (50 g% bread) in healthy young adult mice. The growth curves of all mice examined showed a constant weight gain during the study period of 28 days (Fig. 1). The start weight of the mice was between 17 and 19 g, and after 28 days they weighed between 19 and 22 g. Daily food consumption per mouse was relatively constant between 2.6 and 3.1 g during the 28 days of the experiment. Daily food consumption and weight gain was similar to that in mice fed a standard chow (data not shown).
Growth curves in normal mice. Body weight changes in healthy adolescent mice fed a diet containing 50 g% of yeast-fermented wheat bread from superfine flower defined in Table 1 over 4 weeks. Data are shown as means ± SEM (n = 8)
Survival and Body Weight Change in Response to Different Breads in Caspase-8 Mice
In the next set of experiments, Casp8ΔIEC mice and Casp8fl control mice of the same genetic background (C57BL/6) were fed six study diets described in Table 1 and 2. Depending on the diet the mice received, a variable portion of Casp8ΔIEC mice, but not a single Casp8fl control mouse, died during the study. Survival was 100% in Casp8ΔIEC mice fed with a gluten- and bread-free control diet, and 88% in mice fed with a control diet supplemented with gluten. Survival further decreased to 38–75% depending on the type of bread ingested during the study (Fig. 2A). In total, 20 out of 64 Casp8ΔIEC mice did not reach the regular end of the study, either because they died or because they must be killed prematurely since they developed major symptoms such as severe weight loss, rectal bleeding and/or diarrhea. The worst outcome was observed in mice fed yeast-fermented superfine flour rye bread (38% survival, P = 0.049 compared to GF), but also yeast-fermented spelt and wheat bread were harmful (43% survival for spelt, P = 0.150; 63% survival for wheat SF and WG, P = 0.376 compared to GF). Neither the genotype (Casp8fl and Casp8ΔIEC) nor the type of diet influenced the daily food intake, which varied between 2.4 and 2.9 g/day, or the liver/body weight ratio, which varied slightly between 3 and 5% (Table S2). Growth curves were similar in Casp8ΔIEC survivors and Casp8fl mice (Fig. 2B + C) but became flatter when including all Casp8ΔIEC mice (Table S2). Only the group that received GF had an almost normal weight gain. Body weight changes were variable at the individual mouse level strongly correlated with the genotype (P < 0.001). Also, the diet influenced body weight gain (P = 0.02). Post hoc analyses revealed differences in weight changes between GF compared to all bread-fed mouse groups except W SF SB (Table S2).
Survival and growth curves in ileocolitis and control mice. A Kaplan–Meier survival curves and survival rates of Casp8ΔIEC mice that were fed either a rice-based gluten free diet (GF), rice-based diet with 5% gluten (5G) or a diet containing 50 g% of six different breads listed in the figure legends. Survival of Casp8fl control mice was always 100% and not affected by the different diets (data not shown). Survival of Casp8ΔIEC mice was reduced to 38–88% within 4 weeks depending on the diets offered, but remained 100% in mice fed the GF control diet. There was a significant difference between GF and the rye bread diet (P = 0.049). B + C Growth curves of Casp8ΔIEC survivors (n = 3–7 per group) and Casp8fl mice (n = 8 per group) fed with different diets listed in the figure legend. Each curve shows the mean body weights at given time points for mice of different diet groups. Only data of mice that survived until day 28 are shown
Intestinal Inflammation
Selective endoscopic imaging revealed severe inflammatory lesions throughout the mucosa in some Casp8ΔIEC mice (Fig. 3A). Colonic tissue sections showed variable mucosal damage and neutrophil infiltration into the mucosa (Fig. 3B). Scoring the histological changes revealed that Casp8ΔIEC mice generally had higher inflammation scores than Casp8fl mice, the differences being more pronounced in the colon than in the small intestine (Figs. 3C + D). In general, the scores for inflammation in the ileum were quite low for both Casp8ΔIEC and Casp8fl mice with no diet-related differences (Fig. 3C). In Casp8ΔIEC mice, the extent of colitis was much higher compared to control mice, and different between the six diet groups (Fig. 3D). Two-way-ANOVA revealed genotype-related differences regarding the extent of colitis and identified particular diets aggravating the colitis such as yeast-fermented rye and wheat bread as well as sourdough fermented wheat bread as indicated by hashes (Fig. 3D). Also, ileitis was affected by the genotype (P = 0.03), albeit weakly, without clear discrimination between the diet groups.
Inflammation and tissue damage in Casp8ΔIEC and Casp8fl mice after feeding different bread-rich diets. A Mucosal image derived from colonoscopy showing a severely inflamed colon specimen (high grade, score 6, see methods), the black arrows indicate mucosal areas with ulcerations. B Representative photomicrographs of colonic tissues stained with hematoxylin and eosin; examples for low (score 0–2), middle (score > 2–4), and high (score > 4–6) grade histological scores are shown. C, D Results from quantitative scoring of inflammation and tissue damage in the ileum (C) and the colon (D). Tissue specimen derived from Casp8ΔIEC and Casp8fl mice fed 8 diets each, as described in Table 1. Abbreviations: GF, Gluten free diet; 5G, diet supplemented with 5 g% gluten; W, wheat; SF, superfine flour; Y, yeast fermented; S, sourdough fermented; SB, sourdough fermented with bread improver; Sp, spelt; R, rye). Statistics: Means ± SEM are shown. Two-way ANOVA was conducted to separate genotype effects, diet effects, and possible interaction between genotype and diet. The results are indicated by text in the upper left corner of panels C and D. If e.g. “Genotype” or “Diet” had a significant effect, we performed the Tukey’s multiple comparison test and indicated the results by asterisk on top of the bars for diet effects, or by hash’s on bottom of the bars for genotype effects. */#P < 0.05; **/##P < 0.01, ***/###P < 0.001
Markers of Intestinal Permeability
The expression of tight junction (TJ) proteins, well-recognized as markers of gut barrier function, was measured in the colonic mucosa to further study the role of caspase-8 knockout and diet on gut barrier function. No genotype-specific differences could be seen regarding ZO1, CLDN2 and occludin mRNA expression, while the diet had a great influence (two-way-ANOVA, P < 0.001). No differences could be observed between 5G and GF diet, while TJ protein expression could be shown to be reduced in all mice fed with sourdough wheat bread (W SF S) compared to control diet (in selected cases) and 5G diet (in all mice). TJ protein expression were also reduced in mice fed with sourdough wheat bread compared to mice fed with yeast wheat bread (W SF Y) suggesting that not only wheat but also the type of formation plays a role. When comparing wholegrain with superfine yeast-fermented wheat bread, wholegrain bread resulted in a lower expression of ZO1 and CLDN2 mRNA. No differences could be found when yeast fermented breads from superfine flour of wheat (W), spelt (Sp) and rye (R) were compared to each other (Fig. 4). However, the expression of selected TJs was reduced in mice fed rye bread compared to the 5G diet.
Relative mRNA expression of tight junction proteins in the colon. Data from Casp8fl (panels A–C) and Casp8ΔIEC (panels D–F) mice fed 8 diets each, as described in Table 1 are shown as means ± SEM. Abbreviations see Fig. 3. Statistics by two-way ANOVA as described for Fig. 3. Genotypic differences between Casp8fl and Casp8ΔIEC fed the same diet were not found. Dietary differences within the same genotype are indicated by asterisks. Differences between one of the breads and the control diets are indicated by triangles for GF, and by hashes for 5G * ∇ #P < 0.05; ** ∇∇ ##P < 0.01, *** ###P < 0.001. Abbreviations: ZO1, zonula occludens-1; CLDN2, claudin-2. Other abbreviations see Fig. 3.
Measuring FITC-D-4000 concentration in the plasma as marker for the small intestine barrier function, no differences could be found, neither between the different diet groups nor between Casp8fl and Casp8ΔIEC mice (Figure S1).
Markers of Intestinal Integrity and Bacterial Translocation
The expression of the mucus gene Muc2 and the proinflammatory TNFα gene were measured in the colon, while the expression of LPS associated proteins LBP [24], TLR4 [25] and MYD88 [26] gene expression were analyzed in the liver of the mice. LBP was additionally found to be elevated in the serum of NCWS patients [5]. Gene expression of Muc2 and TNFα in the colon as well as LBP in the liver were strongly related to the genotype (P < 0.001). Only for Muc2 also an influence of the diet could be found after comparison for multiple testing (P = 0.002). The dietetic effect was most pronounced, when comparing sourdough bread with GF (P = 0.008), or when comparing sourdough bread with sourdough bread supplemented with bread improver (P = 0.018) in control mice (Fig. 5).
Heatmap for the expression of gut barrier-related genes. Relative mRNA expression of MYD88, TLR4 and LBP in the liver, and of Muc2 and TNFα in the colon of Casp8fl and Casp8ΔIEC mice is indicted by two color scales, one for TNFα, one for all other genes. Statistics by two-way ANOVA as described for Fig. 3. Abbreviations: MYD88, myeloid differentiation primary response; TLR4, Toll-like receptor 4; LBP, lipopolysaccharide-binding protein; Muc2, mucin-2; TNFα, tumor necrosis factor-α; n.s., not significant. Other abbreviations see Fig. 3. *Posthoc analysis revealed significant differences between GF and W SF SB (P = 0.008) and between WSF S and W SF SB (P = 0.018)
Correlations
Our data revealed a positive correlation between survival of the mice and both Muc2 mRNA expression (rs = 0.332, P = 0.003) in the colon and body weight gain (rs = 0.624, P < 0.001). Moreover, an inverse association between survival of the mice and both TNFα mRNA in the colon (rs = − 0.366, P = 0.001) and LBP mRNA in the liver (rs = − 0.415, P = 0.001) was found (Fig. 6).
Correlations between survival and biomarkers or body weight, respectively. Survival time is indicated on the x-axis, LBP, Muc2 and TNFα gene expression (panels A–C) and body weight (panel D) in the y-axis. Regression curves, the Spearman’s rank correlation coefficients (rs) and the P values are indicated. Abbreviations: see Fig. 5
Discussion
Our mouse studies show that healthy mice not only accept a diet with a high bread portion, containing 50 g% bread but also digest it and develop a constant weight gain. These data confirm previous studies on feeding bread to mice [27,28,29]. The situation changes dramatically, when bread is offered to unhealthy mice suffering from genetically induced colitis. Loss of the caspase-8 gene in mice has been shown to result in a phenotype characterized by epithelial damage and an impaired gut barrier resulting in ileitis and colitis [18, 22]. When administering diets containing 50 g% of bread, we observed pronounced harmful effects varying depending on the bread type. Bread intake was associated with body weight loss and increased mortality in Casp8ΔIEC mice but not in control mice. The mechanisms were not related to the amount of food intake, but to the type of food intake, since harmful events were only seen in mice fed bread, neither in mice fed bread free, nor in mice fed bread free but supplemented with gluten. The harmful effects were also related to the genotype of mice since only Casp8ΔIEC mice died following bread ingestion.
The Casp8ΔIEC genotype causes gut barrier dysfunction and malabsorption resulting from colitis as shown earlier [18, 22]. The central role of gut barrier dysfunction is underlined by our finding of an increased hepatic expression of LBP in Casp8ΔIEC mice compared to Casp8fl mice and a negative correlation between hepatic LBP and survival strongly suggesting that systemic LPS translocation from the intestine to the liver contributed to the enhanced mortality of Casp8ΔIEC mice fed bread. The negative correlation between survival and colonic inflammation scores (rs = − 0.529; P < 0.001, data not shown) further supports our hypothesis that gut barrier dysfunction contributes to the deleterious effects of bread in Casp8ΔIEC mice, since colonic inflammation is associated with bacterial translocation resulting from intestinal epithelium damage [22, 30]. Our observation that mRNA expression of TJ molecules did not differ between Casp8ΔIEC and Casp8fl mice does not necessarily argue against our hypothesis, because epithelial damage can occur also independently from changes in TJ protein expression, if inflammation directly induces necroptosis of enterocytes [18, 31]
Previous studies of our group have shown that Casp8ΔIEC mice spontaneously develop colitis triggered by luminal signals, e.g., bacterial signals derived from the commensal gut microbiome [32, 33]. The present study indicates that not only bacterial components but also components from bread can trigger colitis and possibly ileitis, the latter being minor in the present experiments compared to previous results from our group [22]. The less pronounced ileitis could be due to the lower age of the mice in the present experiments or other experimental conditions.
Which bread components enhance mucosal inflammation in Casp8ΔIEC mice cannot be fully answered, but our data indicate that gluten does not act as a trigger here. Casp8ΔIEC mice fed a bread-free diet (GF), or a bread-free diet supplemented with gluten at an even higher amount than that in the breads, did not show such deleterious effects, even though gluten worsens intestinal barrier function [34,35,36] and induces inflammation in celiac disease and possibly other gastrointestinal diseases [37].
Regarding the different bread types in more detail, rye bread was unexpectedly harmful in Casp8ΔIEC mice regarding weight gain, survival and colitis. A particular factor that could cause the pronounced impairment of body weight gain and the reduced survival in mice fed rye bread could be small molecules such as phytic acid which is found in higher amounts in rye compared to wheat and spelt [38] and is able to reduce the absorption of minerals such as iron, zinc and calcium, and thereby promotes nutritional deficiencies [39,40,41].
Another obviously relevant factor for the harmful effects of breads in in Casp8ΔIEC mice is yeast, since yeast-fermented breads (rye > spelt > wheat) caused less survival and less body weight gain compared to sourdough breads. It has been suggested that in Casp8ΔIEC mice a distinct local microbiota might drive regional inflammation via activation of TLRs or indirectly through the release of cytokines [18, 31]. Possibly, yeast fermented breads modulate the intestinal microbiota in Casp8ΔIEC mice in a way leading to a more pronounced colonic inflammation. Indeed, grain components of potential relevance for microbiota modulation such as FODMAPs [42] or cereal proteins like ATIs [7, 43], lectins [44, 45], and gliadins [46, 47], which have been shown to worsen intestinal gut inflammation, can be degraded to a higher degree by sourdough fermentation compared to yeast fermentation [16, 48,49,50].
However, when analyzing the breads regarding these potential triggers we could show that the amount of mono- and disaccharides was the same, and that the amount of oligosaccharides was only slightly different between the breads without association to inflammation or survival. In addition, in a previous proteome analysis we could show no clear difference regarding the amount of the above-mentioned proteins or other potential inflammatory proteins between yeast and sourdough fermented breads, or between wheat and spelt breads, while rye bread had the lowest overall content of such components compared to wheat and spelt breads [14]. Thus, it is unlikely that these compounds were major contributors to inflammation or mortality in Casp8ΔIEC mice.
Our data suggest yet unknown inflammatory molecules in yeast-fermented bread not detected via proteome or FODMAP analysis that mediate the negative effects of yeast-fermented breads. Alternatively, yeast-fermented bread might lack some protective factors such as inactivated bacterial cells or bacterial metabolites that are generated during bacterial fermentation and have health-promoting effects via mucosal healing and immunomodulatory, anti-inflammatory or antibacterial properties [51, 52].
The difference between sourdough and yeast fermentation could be even more pronounced if fermentation time would be prolonged from about 2 h in our study to about 16 h. Overall, the rather small difference in harmful effects of different breads seen in our study, could be also due to the fact that the strong genotype-related pathologies that occurred in Casp8ΔIEC may have masked some subtle differences between the diets.
In conclusion, our study shows that healthy mice tolerated bread well, while bread-rich diets resulted in serious harmful effects in Casp8ΔIEC mice. In such mice characterized by intestinal inflammation and loss of barrier function, bread-rich diets caused an aggravation of inflammation and loss of barrier function, reduced weight gain and increased mortality. The harmful effects of the breads occurred to a variable degree depending on the bread type, while gluten supplementation without bread was generally well tolerated. Candidates other than gluten like proinflammatory peptides and small bioactive molecules in bread must be considered to influence intestinal barrier impairment and inflammation.
References
Fasano A, Sapone A, Zevallos V, Schuppan D. Nonceliac gluten sensitivity. Gastroenterology. 2015;148:1195–1204.
Catassi C, Gatti S, Lionetti E. World perspective and celiac disease epidemiology. Dig Dis. 2015;33:141–146.
Fasano A, Berti I, Gerarduzzi T et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292.
Battais F, Richard C, Jacquenet S et al. Wheat grain allergies: an update on wheat allergens. Eur Ann Allergy Clin Immunol. 2008;40:67–76.
Uhde M, Ajamian M, Caio G et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut. 2016;65:1930–1937.
Sapone A, Lammers KM, Casolaro V et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23.
Zevallos VF, Raker V, Tenzer S et al. Nutritional Wheat Amylase-Trypsin Inhibitors promote intestinal inflammation via activation of myeloid cells. Gastroenterology. 2017;152:1100–1113.e12.
Limketkai BN, Sepulveda R, Hing T et al. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand J Gastroenterol. 2018;53:147–151.
Shah A, Walker M, Burger D et al. Link between Celiac Disease and Inflammatory Bowel Disease. J Clin Gastroenterol. 2019;53:514–522.
Williams JJ, Kaplan GG, Makhija S et al. Microscopic colitis-defining incidence rates and risk factors: a population-based study. Clin Gastroenterol Hepatol. 2008;6:35–40.
Herfarth HH, Martin CF, Sandler RS, Kappelman MD, Long MD. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1194–1197.
Aziz I, Branchi F, Pearson K, Priest J, Sanders DS. A study evaluating the bidirectional relationship between inflammatory bowel disease and self-reported non-celiac gluten sensitivity. Inflamm Bowel Dis. 2015;21:847–853.
Ellis A, Linaker BD. Non-coeliac gluten sensitivity? The Lancet. 1978;311:1358–1359.
Zimmermann J, Hubel P, Pfannstiel J et al. Comprehensive proteome analysis of bread deciphering the allergenic potential of bread wheat, spelt and rye. J Proteomics. 2021;247:104318.
El Mecherfi KE, Todorov SD, Cavalcanti de Albuquerque MA et al. Allergenicity of fermented foods: Emphasis on seeds protein-based products. Foods. 2020;9:792.
Rizzello CG, de Angelis M, Coda R, Gobbetti M. Use of selected sourdough lactic acid bacteria to hydrolyze wheat and rye proteins responsible for cereal allergy. Eur Food Res Technol. 2006;223:405–411.
Davis PJ, Smales CM, James DC. How can thermal processing modify the antigenicity of proteins? Allergy. 2001;56:56–60.
Günther C, Martini E, Wittkopf N et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31.
van Overbeek FM, Uil-Dieterman IG, Mol IW et al. The daily gluten intake in relatives of patients with coeliac disease compared with that of the general Dutch population. Eur J Gastroenterol Hepatol. 1997;9:1097–1099.
Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–2904.
Kaden-Volynets V, Günther C et al. Deletion of the Casp8 gene in mice results in ileocolitis, gut barrier dysfunction, and malassimilation, which can be partially attenuated by inulin or sodium butyrate. Am J Physiol Gastrointest Liver Physiol. 2019;317:G493–G507.
Volynets V, Reichold A, Bárdos G et al. Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ mice. Dig Dis Sci. 2016;61:737–746.
Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infect. 2006;8:946–952.
Poltorak A, He X, Smirnova I et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088.
Liu S, Gallo DJ, Green AM et al. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433–3442.
Carcea M, Turfani V, Narducci V et al. Bread for the aging population: The effect of a functional wheat-lentil bread on the immune function of aged mice. Foods. 2019;8:510.
Derbise A, Echenique-Rivera H, Garcia-Lopez M et al. Bread feeding is a robust and more physiological enteropathogen administration method compared to oral gavage. Infect Immun. 2020;88:e00810–e819.
Arias M, Cobo M, Jaime-Sánchez P et al. Gut microbiota and systemic inflammation changes after bread consumption: The ingredients and the processing influence. J Funct Foods. 2017;32:98–105.
Guo Y, Zhou G, He C, Yang W, He Z, Liu Z. Serum Levels of Lipopolysaccharide and 1,3-β-D-Glucan Refer to the Severity in Patients with Crohn’s Disease. Mediators Inflamm. 2015;2015:843089.
Günther C, Buchen B, He G-W et al. Caspase-8 controls the gut response to microbial challenges by Tnf-α-dependent and independent pathways. Gut. 2015;64:601–610.
Stolzer I, Kaden-Volynets V, Ruder B et al. Environmental microbial factors determine the pattern of inflammatory lesions in a murine model of Crohn’s Disease-like inflammation. Inflamm Bowel Dis. 2020;26:66–79.
Volynets V, Louis S, Pretz D et al. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a western-style diet or drinking water supplemented with fructose. J Nutr. 2017;147:770–780.
Wagner SJ, Schmidt A, Effenberger MJP et al. Semisynthetic diet ameliorates Crohn’s disease-like ileitis in TNFΔARE/WT mice through antigen-independent mechanisms of gluten. Inflamm Bowel Dis. 2013;19:1285–1294.
Drago S, El Asmar R, Di Pierro M et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41:408–419.
Sander GR, Cummins AG, Henshall T, Powell BC. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005;579:4851–4855.
Sapone A, Bai JC, Ciacci C et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13.
Michniewicz J. Water-Insoluble Pentosans of Wheat: Composition and Some Physical Properties. Cereal Chem. 1990;5:434–439.
Oberleas D, Muhrer ME, O’Dell BL. Effects of Phytic Acid on Zinc Availability and Parakeratosis in Swine. J. Anim. Sci. 1962;21:57–61.
Torre M, Rodriguez AR, Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr. 1991;30:1–22.
Lopez HW, Duclos V, Coudray C et al. Making bread with sourdough improves mineral bioavailability from reconstituted whole wheat flour in rats. Nutrition. 2003;19:524–530.
Zhou S-Y, Gillilland M, Wu X et al. FODMAP diet modulates visceral nociception by lipopolysaccharide-mediated intestinal inflammation and barrier dysfunction. J Clin Invest. 2018;128:267–280.
Pickert G, Wirtz S, Matzner J et al. Wheat Consumption Aggravates Colitis in Mice via Amylase Trypsin Inhibitor-mediated Dysbiosis. Gastroenterology. 2020;159:257–272.e17.
Gong T, Wang X, Yang Y et al. Plant Lectins Activate the NLRP3 Inflammasome To Promote Inflammatory Disorders. J Immunol. 2017;198:2082–2092.
Pusztai A. Characteristics and consequences of interactions of lectins with the intestinal mucosa. Arch Latinoam Nutr. 1996;44:10S–15S.
Harris KM, Fasano A, Mann DL. Cutting edge: IL-1 controls the IL-23 response induced by gliadin, the etiologic agent in celiac disease. J Immunol. 2008;181:4457–4460.
Zhang L, Andersen D, Roager HM et al. Effects of Gliadin consumption on the Intestinal Microbiota and Metabolic Homeostasis in Mice Fed a High-fat Diet. Sci Rep. 2017;7:44613.
de Angelis M, Rizzello CG, Scala E et al. Probiotic preparation has the capacity to hydrolyze proteins responsible for wheat allergy. J Food Prot. 2007;70:135–144.
Huang X, Schuppan D, Rojas Tovar LE et al. Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity. Foods. 2020;9:943.
Loponen J, Gänzle MG. Use of Sourdough in Low FODMAP Baking. Foods. 2018;7:96.
Thakur BK, Saha P, Banik G et al. Live and heat-killed probiotic Lactobacillus casei Lbs2 protects from experimental colitis through Toll-like receptor 2-dependent induction of T-regulatory response. Int Immunopharmacol. 2016;36:39–50.
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS et al. Postbiotics: An evolving term within the functional foods field. Trends in Food Science & Technology. 2018;75:105–114.
Acknowledgment
We thank Christoph Becker and his group from the University of Erlangen, Germany, for providing the Caspase-8 mouse line.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded in part by the Industrial Collective Research (IGF) program of the German Ministry of Economics and Energy (BMWi) managed by the FEI (Research Association of the German Food Industry) and supported by the AiF (German Federation of Industrial Research Associations) (AIF 20763 N to S.C.B.)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. SCB conceived and designed research; JZ, LDF and VK-V performed experiments; BH produced breads; JZ analyzed data and prepared figures; JZ and SCB interpreted results of experiments and drafted manuscript; all authors approved final version of manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest with regard to the present paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An editorial commenting on this article is available at https://doi.org/10.1007/s10620-022-07466-z.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zimmermann, J., De Fazio, L., Kaden-Volynets, V. et al. Consumption of Yeast-Fermented Wheat and Rye Breads Increases Colitis and Mortality in a Mouse Model of Colitis. Dig Dis Sci 67, 4422–4433 (2022). https://doi.org/10.1007/s10620-022-07462-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07462-3