Abstract
Background and Aims
Feed intolerance (FI) is common in cirrhosis patients in intensive care units (ICU). Prokinetics are the first line treatment for FI but their efficacy and safety in critically ill patient with cirrhosis is unknown. We evaluated the role of prokinetics in reversal of FI and clinical outcomes.
Methods
Consecutive patients admitted in ICU developing new-onset FI, were randomized to receive either intravenous metoclopramide (Gr.A, n = 28), erythromycin (Gr.B, n = 27) or placebo (Gr.C, n = 28). FI was defined with the presence of 3 of 5 variables- absence of bowel sounds, gastric residual volume ≥ 500 ml, vomiting, diarrhoea and bowel distension. Primary end-point was complete resolution of FI (≥ 3 variables resolved) within 24-h and secondary end-points included resolution within 72-h and survival at 7-days.
Results
Of the 1030 ICU patients, 201 (19.5%) developed FI and 83 patients were randomized. Baseline parameters between the groups were comparable. Complete resolution at 24-h was higher in Gr.A (7.14%) and B (22.2%) than C (0%, p = 0.017). Overall, 58 (69.9%) patients achieved resolution within 72 h, more with metoclopramide (n = 24, 85.7%) and erythromycin (n = 25, 92.6%) than with placebo (n = 9, 32.1%, p < 0.001). The 7-day survival was better in patients who achieved resolution within 72-h (65.5 vs. 36%, p = 0.011) than non-responders. High lactate (OR-3.32, CI-1.45–7.70, p = 0.005), shock at baseline (OR-6.34, CI-1.67–24.1, p = 0.007) and resolution of FI within 72 h (OR-0.11, CI, 0.03–0.51, p = 0.04) predicted 7-day mortality.
Conclusions
FI is common in critically-ill cirrhosis patients and non-resolution carries high mortality. Early recognition and treatment with prokinetics is recommended to improve short-term survival.
Lay Summary
Gastrointestinal dysmotility is common in cirrhosis and higher incidence in critically ill patients. Promotility drugs are the first line of medication especially in ICU patients. In our study, we found that feed intolerance is present in nearly one in five critically ill cirrhosis and is associated with higher mortality. Patients who achieve resolution had an improved short-term survival. Prokinetic medications are safe in critically ill cirrhosis and help in early resolution of feed intolerance. Feed intolerance in critically ill cirrhosis should be recognized as an organ dysfunction and approaches for prevention and early diagnosis of feed intolerance could help in improving the outcomes in critical illness.
Similar content being viewed by others
Introduction
Gastrointestinal symptoms (GI) are common in patients admitted in intensive care unit (ICU) occurring in around 60% of patients [1]. GI dysmotility may occur in stomach or, small or large intestine, causing delayed gastric emptying, feed intolerance or ileus [2]. Autonomic dysfunction, delayed gastric emptying [3], and prolonged small bowel transit [4] are common in cirrhosis, adding to the increase in development of gastroparesis and cessation of enteral nutrition. Nutrition is compromised in cirrhotic patients, with upto 60% of Child C cirrhotic patients having moderate to severe degree of protein-energy malnutrition (PEM) [5]. Critical illness and sepsis add to the overall energy deficient state. The incidence of feed intolerance is seen in around 30% in patients admitted to ICU [6] but a clear-cut defining criterion is lacking for diagnosing feed intolerance (FI) and is usually defined based on volume of gastric residues or GI symptoms. A retrospective study compared different definitions for feed intolerance, based on clinical symptoms, gastric residual volume (GRV), and/or calorie intake, and concluded correlation with ICU mortality based on at least 3 out of 5 variables, namely, absence of bowel sounds, vomiting or regurgitations, diarrhea, bowel distension and large gastric residual volumes of > 500 ml/day [7]. Although, an important criterion for defining FI, a large open label trial reported that non-measurement of GRV was not inferior to routine residual volume monitoring in prevention of other complications like ventilator associated pneumonia [8].
Enteral feeding is the preferred mode of feeding in ICU patients. Guidelines recommend against the early administration of parenteral nutrition, especially during the first 7 days [9], but also state to hold the enteral feeding in critically ill patients with uncontrolled shock, severe hypoxemia or acidosis, uncontrolled upper GI bleed, gastric aspirate of more than 500 ml, surgical abdomen, abdomen compartment syndrome, or fistula with high-output [10].
Promotility drugs, metoclopramide, a dopamine receptor antagonist, and erythromycin, a motilin receptor agonist, are the first line agents for feed intolerance with intravenous erythromycin considered as drug of choice, for feed intolerance especially in critically ill patients [10]. Studies in non-cirrhotic patients has shown response, ranging between 62–85% with promotility drugs, with maximal effect in initial 24 h and also suggest for combination therapy for improved response [12, 13]. Meta-analysis has shown a 17.3% absolute reduction in feed intolerance with use of promotility drugs [14]. Different doses of erythromycin have been tried with equivalent response reported with 70 and 200 mg doses of intravenous erythromycin compared to placebo when assessed based on gastric emptying co-efficient (GEC) using 13C octanoic acid breath test [15]. Although data is available in usage and efficacy of prokinetics in feed intolerance in non-cirrhotic patients, similar data is lacking in critically ill cirrhosis. We undertook a prospective randomized study assessing the burden of feed intolerance in critically ill patients with cirrhosis and hypothesized that feed intolerance is a part of organ failure and need early resolution.
Patients and Methods
Study Design
This was an open label, placebo controlled randomized trial conducted at the Department of Hepatology, Institute of Liver and Biliary Sciences (ILBS), New Delhi, India from August 2015 to August 2017. This single centre protocol confirmed to the Declaration of Helsinki and was approved by institutional ethics committee with letter number- F-25/5/80/ILBS/AC/2015/733 and followed the CONSORT guidelines for randomized controlled trial. This study was registered at ClinicalTrials.gov with identifier number- NCT02528760. All the authors had contributed and had access to the data.
Methodology
All liver disease patients consecutively admitted in ICU were screened and those who developed feed intolerance were enrolled. All patients underwent investigations and treatment as per institutional ICU protocol. They were enrolled in the study if a new onset of feed intolerance developed during the course of ICU stay. Correctable factors, including electrolyte imbalance, presence of tense ascites were attended to, and patients were randomized into the treatment arm if feed intolerance persisted. Antibiotic were upgraded and cultures sent in cases with suspected infections. Clinical findings and symptoms were checked and noted every 6 hourly. Feed intolerance was defined as per study definition of atleast 3 out of 5 positive variables with one variable of gastric residual volume necessary for defining FI. The variables included, absence of bowel sounds defined as no bowel sound heard during auscultation, vomiting defined as visible regurgitation of gastric content, diarrhoea defined as passing loose stools for more than 3 times per day, bowel distension defined as visible abdominal distension or subjective feeling and confirmed on x ray with small bowel > 3 cm or large bowel > 5 cm and large gastric residual volume (GRV) defined as an aspirate of ≥ 500 ml of gastric volume over 24 h. Gastric residual volumes (GRV) was checked at every 4–6 hourly interval. GRV was measured by either gravity drainage by connecting a gastric tube to a drainage bag for 10 min or by manual aspiration of content using a 50 ml syringe. Once feed intolerance developed, intra-abdominal pressure monitoring and abdominal girth measurement was done every 6 hourly. Intra-abdominal pressure measurement was done by Foley’s manometer technique. A portable X-ray abdomen supine was obtained to look for bowel distension, defined as more than 3 cm for small bowel and more than 5 cm in large bowel. Patients were considered for CT scan (with or without iv or oral contrast) as per clinical and hemodynamic stability and renal parameter status. Patients were randomized into three groups; either to receive intravenous (iv) metoclopromide 10 mg every 8 hourly [14], iv erythromycin 70 mg every 12 hourly [15] or normal saline in 10 ml syring iv twice daily as placebo. Initial response to therapy was assessed at 24 h duration after starting of treatment in each arm. Complete resolution was defined as resolution of at least 3 out of 5 of the symptom variables with definite resolution of large GRV, and re-initiation of enteral nutrition. Therapy was continued for a total duration of 72 h and patients were followed-up for a maximum of 7 days. Primary end-point was response at 24 h and secondary end-points were resolution within 72 h or death within 7 days. Rescue treatment in the form of post-pyloric tube placement, combination of treatment or if required, endoscopic colonic decompression, was considered after initial 24 h based on clinical response.
Eligibility Criteria
All patients of chronic liver disease and of age ≥ 18 and ≤ 70 years who were admitted in ICU were prospectively screened. Patients of cirrhosis and with decompensation as well as those with acute on chronic liver failure (ACLF), as defined by Asia Pacific association for the study of the liver (APASL) definition were prospectively enrolled [16]. Patients who were requiring dual vasopressor, on high vasopressors (> 0.64 mcg/kg/min), uncontrolled sepsis, DIC, and patients with GI bleed at the time of development of feed intolerance were excluded. Current or past history of surgical abdomen, including mechanical obstruction, mesenteric ischemia, perforation or requiring abdominal surgery, patients receiving nutrition through gastrostomy or feeding jejunostomy, prior comorbid illnesses like advanced cardiopulmonary disease, prior episodes of arrhythmia, structural heart diseases, traumatic brain injury, cerebrovascular accidents, raised intracranial pressures, history of myasthenia gravis, endocrinological illnesses like uncontrolled hypothyroidism, hypoparathyroidism, previously diagnosed diabetic gastroparesis, connective tissue disorders including systemic sclerosis, dermatomyositis or polymyositis, systemic lupus erythematosus, amyloidosis were excluded. Patients were excluded if they had received prokinetic medication during ICU stay or were allergic to the medications (either metoclopramide or erythromycin), pregnant females and refusal to give consent.
Objectives
The primary objective was to determine the role of prokinetics at 24 h of initiation of treatment in critically ill cirrhotic patients who develop new onset of feed intolerance. Secondary objectives included the incidence of FI, mean duration of ICU stay for development of FI, resolution of FI at 72 h, safety of prokinetics in CIC, requirement of rescue treatment in non-responders, risk factors associated with the development of FI and survival at 7 days. End point was resolution of 3 out of 5 criteria of feed intolerance and mortality till day 7. Patients were followed up till death or discharge from the ICU.
Sample Size and Randomization
Based on previous studies in non-cirrhotic population with response rate of reversal of feed intolerance and re-initiation of enteral feeding at 24 h patients seen in 87% receiving erythromycin and that of metoclopramide was around 62% [12], and assuming placebo effect of around 5% affect, with alpha of 5% and power of study to be 90%, 49 patients were to be enrolled in each arm. With assumption of around 10% defaulter rate, it was decided to enrol 54 patients in each group. Patients were randomized using computer generated block randomization with a block size of 15 and using sequentially numbered opaque sealed envelope (SNOSE) technique.
Statistics
Baseline data expressed as a proportion, either mean ± SD or median (IQR). The statistical technique applied were one way ANOVA/Kruskal Wallis test as appropriate to compare between groups followed by Post hoc comparison was performed by Bonferroni method. The categorical data was analysed using χ2 test or Fisher’s exact test. Significance was defined as 2-tailed p value of less than 0.05. Univariate and multivariate logistic regression were used to see the predictors of non-resolution at 72 h. Kaplan–Meier method was used to plot survival curves. The data was entered in Microsoft excel format and was analysed using SPSS version 22 (IBM corp Ltd.; Armonk NY, USA).
Results
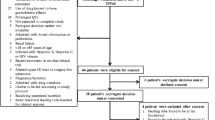
A total of 1030 patients with liver disease were admitted in our intensive care unit during the study period, who fulfilled the inclusion criteria, and were prospectively enrolled and followed up. A total of 201 (19.5%) patients developed new-onset feed intolerance during ICU stay. The mean duration of ICU stay for the development of feed intolerance was 6.65 (IQR, 2–29) days. Feeding was restarted within 24 h after correction of dyselectrolytemia in 14.4% (n = 29) patients and after therapeutic paracentesis, for tense ascites, in 4.97% (n = 10) patients. Seventy nine (39.3%) patients were excluded with uncontrolled sepsis and septic shock (n = 38), dual vasopressor support (n = 27), prior CAD (n = 3), prior CVA (n = 1), mechanical obstruction (n = 3), age > 70 years (n = 2) and failure to give consent for the study (n = 5). Eighty three patients were randomized into three groups to receive either intravenous (iv) metoclopramide (n = 28), (iv) erythromycin (n = 27) or iv Placebo (n = 28), respectively. Baseline parameters were comparable among the groups, with mostly middle aged males and alcohol (n = 42) being the predominant etiology followed by non-alcoholic fatty liver disease (n = 21) cirrhosis. Ascites was present in 79 (95.1%) patients and grade 3 ascites requiring therapeutic paracentesis seen in 23 (29.1%) patients (Fig. 1). Comparison of other baseline parameters in-between the groups is given in Table 1.
Incidence and Spectrum of Feed Intolerance
New onset of feed intolerance was seen in 201 (19.5%, 95% CI, 17.1–22.07%) patients admitted in ICU during the study period. The median FI score was 3 (IQR, 3–4). Clinical presentation of feed intolerance was predominantly as gastroparesis with many patients having concomitant paralytic ileus (n = 36, 43.4%). Fifty-three patients (63.9%) underwent abdominal computed tomography (CT) scan with oral contrast, due to non-resolution of feed intolerance within 24 h. Dilated small bowel loops was seen in 29 (54.8%) patients and dilated colonic loops were seen in 6 (11.3%) patients.
Primary Outcome
Complete resolution was better in patients receiving prokinetics as compared to placebo with resolution seen in 8 (9.63%) patients, 2 (7.14%) patients in metoclopramide group and 6 (22.2%) patients in erythromycin group (p = 0.11), respectively. None of the patients receiving placebo achieved resolution within 24 h (p = 0.017).
Secondary Outcomes
Overall, 58 (69.9%) patients achieved complete resolution of feed intolerance within 72 h with higher resolution (p < 0.001) seen in patients receiving metoclopramide (n = 24, 85.7%) and erythromycin (n = 25, 92.6%) as compared to placebo (n = 9, 32.1%), (Table 2). Prokinetics also helped in early re-initiation of feeding (2.86 ± 0.76, 2.15 ± 0.72, 3.43 ± 1.14 days, p = < 0.001). Amongst the two prokinetic groups, feeding was restarted earlier in patients receiving erythromycin as compared to metoclopramide (p = 0.013). The mean change in GRV (ΔGRV) from 24 to 48 h was comparable among groups (p = 0.16) but higher reduction in volumes was seen over 72 h in patients receiving prokinetics (Table 3). Persistent feed intolerance was seen in 12 (14.5%) patients after 72 h of treatment and were considered for rescue treatment. Post-pyloric tube was placed in 9 patients and 3 patients were considered for endoscopic colonic decompression. Two patients initially on standard medical therapy were considered for iv metoclopramide after 72 h for persistent high gastric residual volume along with post pyloric tube placement for feeding.
Adverse Effects
Two patients (7.14%) patients developed headache and one (3.57%) patient noticed dizziness after the dose of metoclopramide, not requiring drug discontinuation. None of the patients developed extrapyramidal symptoms. Three (11.1%) patients had diarrhea and one patient (3.70%) developed sinus tachycardia on day 3 of treatment with erythromycin and was attributed to worsening sepsis. None of the patients had worsening of liver function with significant rise in transaminases, requiring drug withdrawal.
Predictors of Non-resolution
On univariate analysis, requirement of renal replacement therapy (RRT) (odds ratio [OR], 5.98; 95% confidence interval [CI], 1.13–32.25; p = 0.02), treatment with erythromycin compared to placebo (OR, 0.32, CI, 0.10–0.96, p = 0.04) and presence of spontaneous bacterial peritonitis (SBP) at baseline at the time of admission to ICU (OR, 1.14, CI, 1.04–1.26, p = 0.09) was associated with non-resolution of feed intolerance at 24 h. Treatment with metoclopramide (OR, 0.08, CI, 0.02–0.29, p < 0.001) and erythromycin (OR, 0.04, CI, 0.01–0.19, p value < 0.001) compared to placebo were significantly associated resolution at 72 h. On multivariate logistic regression analysis, need of RRT (OR, 11.49, 95%CI, 1.63–83.3, p = 0.014) was independently associated with non-resolution of feed intolerance at 24 h (Table 4).
Predictors of Mortality
On univariate analysis, presence of infection (bacteremia) (OR, 7.42, CI, 1.8–66.6, p = 0.04), high lactate levels (OR, 3.32, CI, 1.71–6.44, p < 0.001), presence of shock at baseline (OR, 3.77, CI, 1.51–9.43, p = 0.004), requirement of renal replacement therapy (OR, 2.64, CI, 1.02–6.81, p = 0.04), mechanical ventilation (OR, 3.84, CI, 1.26–11.71, p = 0.014), high FiO2 requirement (OR, 1.04, CI, 0.98–1.106, p = 0.15), and on propofol (OR, 2.61, CI, 1.06–6.38, p = 0.03) or fentanyl (OR, 3.66, CI, 1.45–9.19, p = 0.005), treatment with erythromycin compared to placebo (OR, 0.31, CI, 0.10–0.96, p = 0.04) and resolution of FI within 72 h (OR, 0.29, CI, 0.11–0.79, p = 0.015) were significantly associated for mortality at 7 days. On multivariate logistic regression, arterial lactate (OR, 3.32, CI-1.45–7.70, p = 0.005), presence of shock at baseline (OR, 6.34, CI-1.67–24.1, p = 0.007), resolution of FI within 72 h (OR-0.11, CI, 0.03–0.51, p = 0.04) were independently associated with mortality at 7 days (Table 5).
Survival Analysis
Short-term survival of 7-days was comparable between the treatment groups (p = 0.21). Of the 83 patients, 36 (43.4%) died; 12 (42.9%) in metoclopramide, 8 (29.6%) in erythromycin and 16 (57.1%) in the placebo group (Fig. 2). However, patients who showed complete resolution of feed intolerance within 72 h had improved survival compared to non-responders (65.5 vs. 36%, p = 0.011) (Fig. 3).
Discussion
The results of this novel randomized controlled trial highlights the significance of gut paralysis in critically ill cirrhosis and shows early resolution of FI improves short-term survival. FI was observed in one in five [19.5%] patients, which resolved with correction of metabolic factors in 14.4% and persisted in 80.6% of patients, despite of correction of factors. Moreover, resolution of FI within 72 h independently showed reduced 7 day mortality (OR-0.11, CI, 0.03–0.51, p = 0.04). Considering resolution of feed intolerance to be an independent predictor of survival, therefore all efforts should be made to hasten its resolution. Amongst the two prokinetics studied, erythromycin was found to be superior to metoclopramide in improving FI [22.2 vs. 7.14%, p = 0.11) and early initiation of enteral feeding (p = 0.013).
The incidence of FI after admission to the ICU was 19.5%. In a multicentre observational study conducted in 1888 patients in ICU [6], the overall incidence was reported as 30.5% and the prevalence of feed intolerance has been reported to vary between 2 and 75%, depending upon the definition used for feed intolerance which varies both in terms of GRV and/or inclusion of GI symptoms [18]. The spectrum of GI manifestations includes abdominal distension, vomiting, high GRV and subjective sense of discomfort as the factors for FI and discontinuation of enteral feeding. Studies on cirrhotic patients have shown that both gastroparesis as well as slow small bowel transit, seen in upto 40% of patients, led to discontinuation of enteral nutrition [4]. In our study, although all the patients had high GRV (> 500 ml/day), many patients (54.7%) had concomitant bowel distension. It was also important to note that in cirrhosis, bowel distension may also be related to factors, including intra-abdominal sepsis, hypoalbuminemia, or grade 3 ascites. Various factors influence the development of feed intolerance in ICU setting with studies showing nearly 51% patients intolerant to enteral nutrition, especially those who were on mechanical ventilation [8]. However, higher proportion of patients were labelled to have feed intolerance based on isolated GRV estimation [8]. In our study, hemodynamic instability and requirement of vasopressors was seen in 45% of patients who were randomized and a nearly one-third of patients were excluded from randomization as they were on high vasopressor support or on dual vasopressors. Presence of shock at baseline was also associated with overall increased 7-day mortality. Previous studies have also shown increased GI complications [19] in critically ill patients, especially those with baseline hemodynamic instability [11].
Feed intolerance is defined usually in terms of GRV and no single cut-off for GRV estimation is defined in ICU settings, with lack of defining criteria for critically ill cirrhotic. Studies have shown a higher GRV (≥ 500 ml) are not associated with higher GI complications [17] compared to lower GRV (< 200 ml). Randomized trials have shown that absence of GRV measurement is non-inferior to routine GRV measurement in ICU setting. The definition for FI need standardization and should be based not only on the basis of GRV but should include clinical symptoms. In our study, we followed the definition given by Reitnam Blaser et al. [7], taking both the GRV and clinical factors into consideration (3 out of 5 variables), which correlated best with ICU mortality in non-cirrhotic ICU patients. We found a 7-day survival benefit in patients who showed early resolution of feed intolerance (within 72 h) as compared to non-resolvers or late resolvers (65.5 vs. 36%, p = 0.011).
There is paucity of data regarding the role of prokinetics in cirrhosis and data is lacking on comparative efficacy of metoclopramide in comparison to erythromycin in CIC. Response to prokinetics is highest during the initial few days and later develop tachyphylaxis [12]. In our study, the definition for resolution was based not on a single entity of GRV, lower overall response in terms of complete resolution was seen during the initial 24 h (9.6%). This was also evident based on gastric residual volumes estimation (Table 3), as groups receiving metoclopramide and erythromycin had median GRV volume of < 500 ml beyond 24 h and cumulative resolution within 72 h, of more than 80% as compared to placebo.
In our study, we found that patients with early resolution had a short-term survival benefit with 65.5% achieving resolution of FI with first 72-h. We believe that as patients with decompensated CLD and those with ACLF have increased systemic inflammation and deranged intestinal barrier functions [20] and leaky gut leads to increased bacterial product translocation into the portal circulation, an early resolution of gut paralysis and restarting enteral feeding prevents the progression of this cascade into disease progression and development of multi-organ failure [21]. This also emphasise the increased occurrence of development of extra-hepatic organ failure, need for ICU admissions and mortality in hospitalized cirrhosis patients with higher dysbiosis on admission [22]. Similarly, decreased microbial diversity and richness was seen in ACLF patients compared to control group resulting in increased mortality [23].
Feed intolerance is a major morbidity in critically ill cirrhosis patients and accounts for cessation of enteral feeding. Our study shows the benefit of prokinetics and its effect in early re-initiation of enteral feeding. Still there were few limitation in our study. Although more than 200 patients developed new onset feed intolerance during the study period, many were excluded and just over 50% of previously planned patients could be enrolled during the study period. Prokinetics given act mainly in the upper GI tract and significantly decreased gastric residual volume. Thus, the reason for resolution and re-initiation of feeding possibly could be related to overall clinical improvement and not just based on prokinetics treatment. We recommend treatment with addition of prokinetics for early resolution and decrease the days of enteral feeding cessation. Our study findings open frontiers for new challenges and treatment in critically ill cirrhosis, along with role of bacterial dysbiosis, as our findings specifically shows survival benefit in FI resolvers.
Abbreviations
- FI:
-
Feed intolerance
- ACLF:
-
Acute on chronic liver failure
- CLD:
-
Chronic liver disease
- CTP:
-
Child-Turcotte-Pugh
- MELD:
-
Model for end stage liver disease
- GRV:
-
Gastric residual volume
References
Reintam A, Parm P, Kitus R et al. Gastrointestinal symptoms in intensive care patients. Acta Anaesthesiol Scand. 2009;53:318–324.
Deane AM, Chapman MJ, Reintam Blaser A et al. Pathophysiology and treatment of gastrointestinal motility disorders in the acutely ill. Nutr Clin Pract. 2009;34:23–26.
Verne GN, Soldevia-Pico C, Robinson ME et al. Autonomic dysfunction and gastroparesis in cirrhosis. J Clin Gastroenterol. 2004;38:72–76.
Kalaitzakis E, Sadik R, Holst JJ et al. Gut transit is associated with gastrointestinal symptoms and gut hormone profile in patients with cirrhosis. Clin Gastroenterol Hepatol. 2009;7:346–352.
Carvalho L, Parise ER. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arg Gastroenterol. 2006;43:269–274.
Gungabissoon U, Hacquoil K, Bains C et al. Prevalence, risk factors, clinical consequences, and treatment of enteral feed intolerance during critical illness. JPEN J Parenter Enteral Nutr. 2015;39:441–448.
Reintam Blaser A, Starkopf L, Deane AM et al. Comparison of different definitions of feeding intolerance: a retrospective observational study. Clin Nutr. 2015;34:956–961.
Reignier J, Mercier E, Le Gouge A et al. Effect of not monitoring residual gastric volumes on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA. 2013;309:249–256.
Rhodes A, Evans LE, Alhazzani W et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377.
Singer P, Blaser AR, Berger MM et al. ESPEN guidelines on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38:48–79.
Reignier J, Boisrame-Helms J, Brisard L et al. NUTRIREA-2 Trial Investigators; Clinical Research in Intensive Care and Sepsis (CRICS) group. Enteral versus parenteral early nutrition in ventilated adults with shock: a randomized, controlled, multicenter, open-label, parallel-group study (NUTRIREA-2). Lancet. 2018;391:133–143.
Nguyen NQ, Chapman MJ, Fraser RJ et al. Erythromycin is more effective than metoclopramide in the treatment of feed intolerance in critical illness. Crit Care Med. 2007;35:483–489.
Hersch M, Krasilnikov V, Helviz Y et al. Prokinetic drugs for gastric emptying in critically ill ventilated patients: Analysis through breath testing. J Crit Care. 2015;30:e7-13.
Lewis K, Algahtani Z, Mcintyre L et al. The efficacy and safety of prokinetic agents in critically ill patients receiving enteral nutrition: a systematic review and meta-analysis of randomized trials. Crit Care. 2016;20:259.
Ritz MA, Chapman MJ, Fraser RJ et al. Erythromycin dose of 70mg accelerates gastric emptying as effectively as 200mg in the critically ill. Intensive Care Med. 2005;31:949–954.
Sarin SK, Kedarisetty CK, Abbas Z et al. Acute-on-chronic liver failure: consensus recommendations of the Asia Pacific Association for the Study of Liver (APASL) 2014. Hepatol Int. 2014;8:453–471.
Montejo JC, Minambres E, Bordeje L et al. Gastric residual volume during enteral nutrition in ICU patients. Intensive care Med. 2010;36:1386–1393.
Reintam Blaser A, Starkopf J, Kirsimagi U et al. definition, Prevalence, and Outcomes of Feeding Intolerance in Intensive Care: A Systematic Review and Meta-Analysis. Acta Anaesthesiol Scand. 2014;58:914–922.
Harvey SE, Parrott F, Harrison DA et al. Trial of the Route of Early Nutritional Support in Critically Ill Adults. N Engl J Med. 2014;371:1673–1684.
Weist R, lawson M, Geuking M. Pathological Bacterial Translocation in Liver Cirrhosis. J Hepatol. 2014;60:197–209.
Alukal J, Thulavath P. Gastrointestinal Failure in Critically Ill Patients with Cirrhosis. Am J Gastroenterol 2019;114:1231–1237.
Bajaj JS, Vargas HE, Reddy KR et al. association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17:756–765.
Chen Y, Guo J, Qian G et al. Gut dysbiosis in acute-on-chronic liver failure and its predictive value for mortality. J Gastroenterol Hepatol. 2015;30:1429–1437.
Author information
Authors and Affiliations
Contributions
Study design—SKS RV RM JB AC KDJ YKJ. Patient recruitment—RV RM SKS. Data acquisition—RV VA. Statistical analysis—SKS RV RM GK. Manuscript drafting—RV SKS RM. Manuscript revision and approval—SKS RV RM VA AC JB PA KDJ YKJ.
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vijayaraghavan, R., Maiwall, R., Arora, V. et al. Reversal of Feed Intolerance by Prokinetics Improves Survival in Critically Ill Cirrhosis Patients. Dig Dis Sci 67, 4223–4233 (2022). https://doi.org/10.1007/s10620-021-07185-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07185-x