Abstract
Background
Optimal management of patients with ulcerative colitis (UC) requires the accurate, objective assessment of disease activity.
Aims
We aimed to determine how strong patient-reported outcomes, clinical scores and symptoms correlate with endoscopy and biomarkers for assessment of disease activity in patients with UC.
Methods
Consecutive patients with UC followed at the McGill University IBD Center and referred for endoscopy (surveillance or flare) were included prospectively between September 2018 and August 2020. Patient-reported outcome (PRO2), partial Mayo, Simple Clinical Colitis Activity Index (SCCAI), Mayo endoscopic subscore (MES) and Baron and Ulcerative Colitis Endoscopic Index of Severity (UCEIS) scores were calculated. C-reactive protein (CRP) and fecal calprotectin (FCAL) were collected.
Results
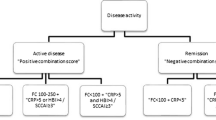
A total of 171 patients with UC [age: 49(IQR:38–61) years, female: 46.2%, 57.3% extensive disease, 42.7% on biologicals] were included prospectively. Rectal bleeding (RBS), stool frequency (SF) subscore of 0, or total PRO2 remission (RBS0 and SF ≤ 1), partial Mayo (≤ 2) and SCCAI (≤ 2.5) remission were similarly associated with mucosal healing defined by MES (0 or ≤ 1), Baron (0 or ≤ 1) or UCEIS (≤ 3) scores in ROC analysis (AUC:0.93–0.72). There was a moderate-to-strong agreement between MES Baron and UCEIS (K = 0.91–0.41). A UCEIS of ≤ 3 was identified as the best cutoff to clinical or endoscopic remission. Agreement between CRP and clinical remission or endoscopic healing (MES/Baron) was poor (K ~ 0.2), while agreement between FCAL and RBS-PRO2 or MES/Baron/UCEIS was moderate to strong (K = 0.44–0.70).
Conclusions
Agreement between RBS, SF, PRO2, partial Mayo and SCCAI in predicting endoscopic healing was moderate to strong, while no clinically meaningful difference was found in accuracy across the scores and definitions. FCAL, but not CRP, was associated to clinical and endoscopic remission.



Similar content being viewed by others
References
De Dombal FT. Ulcerative colitis: definition, historical background, aetiology, diagnosis, naturel history and local complications. Postgrad Med J. 1968;44:684–692.
Ardizzone S, Cassinotti A, Duca P et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483-489.e3.
Ferrante M, Vermeire S, Fidder H et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219–225.
Rutter MD, Saunders BP, Wilkinson KH et al. Cancer surveillance in longstanding ulcerative colitis: endoscopic appearances help predict cancer risk. Gut. 2004;53:1813–1816.
Peyrin-Biroulet L, Sandborn W, Sands BE et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338.
Turner D, Ricciuto A, Lewis A et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies. IBD Gastroenterol 2021;160:1570–1583.
Colombel JF, Keir ME, Scherl A et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017;66:2063–2068.
Jharap B, Sandborn WJ, Reinisch W et al. Randomised clinical study: discrepancies between patient-reported outcomes and endoscopic appearance in moderate to severe ulcerative colitis. Aliment Pharmacol Ther. 2015;42:1082–1092.
Narula N, Alshahrani AA, Yuan Y, Reinisch W, Colombel JF. Patient-reported outcomes and endoscopic appearance of ulcerative colitis-a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17:411–418.
Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the mayo score to assess clinical response in Ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629.
Manginot C, Baumann C, Peyrin-Biroulet L. An endoscopic Mayo score of 0 is associated with a lower risk of colectomy than a score of 1 in ulcerative colitis. Gut. 2015;64:1181–1182.
Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43:29–32.
Restellini S, Chao CY, Martel M et al. Clinical parameters correlate with endoscopic activity of ulcerative colitis: a systematic review. Clin Gastroenterol Hepatol. 2019;17:1265-1275.e8.
Jairath V, Khanna R, Zou GY et al. Development of interim patient-reported outcome measures for the assessment of ulcerative colitis disease activity in clinical trials. Aliment Pharmacol Ther. 2015;42:1200–1210.
Baron JH, Connell AM, Lennard-Jones JE. Variation between observers in describing mucosal appearances in proctocolitis. Br Med J 1964;1:89–92.
Travis SPL, Schnell D, Krzeski P et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535–542.
Jusué V, Chaparro M, Gisbert JP. Accuracy of fecal calprotectin for the prediction of endoscopic activity in patients with inflammatory bowel disease. Dig Liver Dis. 2018;50:353–359.
D’Haens G, Ferrante M, Vermeire S et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224.
Colombel J-F, Keir ME, Scherl A et al. Discrepancies between patient-reported outcomes, and endoscopic and histological appearance in UC. Gut. 2017;66:2063–2068.
Arai M, Naganuma M, Sugimoto S et al. The Ulcerative Colitis Endoscopic Index of Severity is useful to predict medium- to long-term prognosis in ulcerative colitis patients with clinical remission. J Crohns Colitis. 2016;10:1303–1309.
Acknowledgments
The article was presented with preliminary data at the ECCO and UEG week 2020 and with final results at DDW 2021 and ECCO 2021.
Funding
The study was supported by the McGill CAS Research Support Program and the WI244988 Pfizer unrestricted research grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None declared by all co-authors.
Ethical considerations
The study was approved by the McGill University Health Centre institutional reviews board and ethics committee (REB no. 2019–4842). The research protocol conforms to ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and local regulations. Informed consent was obtained from each patient included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10620_2021_7178_MOESM1_ESM.tif
Supplementary file1 Figure 1. Association between PRO2 combined remission and endoscopic healing defined by UCEIS (TIF 50 KB)
Rights and permissions
About this article
Cite this article
Golovics, P.A., Gonczi, L., Reinglas, J. et al. Patient-Reported Outcome and Clinical Scores Are Equally Accurate in Predicting Mucosal Healing in Ulcerative Colitis: A Prospective Study. Dig Dis Sci 67, 3089–3095 (2022). https://doi.org/10.1007/s10620-021-07178-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07178-w