Abstract
Background
Patient-reported outcomes (PROs), such as the short CD activity index (sCDAI) and partial Mayo Score (PMS), are used to define clinical remission in IBD, but may not represent the true degree of inflammation and endoscopy is invasive. Non-invasive testing options include c-reactive protein (CRP) and fecal calprotectin (FCP).
Aim
The aim of this study was to assess the degree of correlation of non-invasive biomarkers with PROs and the impact other clinical variables can have on their levels.
Methods
We reviewed data collected from the prospective cohort, Study of a Prospective Adult Research Cohort with IBD (SPARC-IBD), comprised of over 3000 patients from 17 tertiary referral centers. Demographic and clinical variables were analyzed by disease type, disease severity was based on PROs, and baseline CRP and FCP were measured. For comparative analysis, we performed Fisher’s exact test and Welch’s t test, where p < 0.05 was significant.
Results
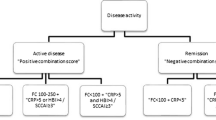
1547 patients were included; 63% had CD, 56% were female, with an average disease duration of 13.6 years. CRP and FCP were associated with symptom severity in inflammatory CD. CRP was useful to differentiate symptoms across different disease locations in CD, whereas FCP was associated with symptom severity in Crohn’s colitis only. For UC, FCP was able to distinguish symptom severity better in distal UC, whereas in extensive or pancolitis, it was useful only to distinguish severe symptoms from other categories of symptom severity.
Conclusion
PROs correlate with CRP and FCP; however, disease location and phenotype impact their ability to distinguish symptom severity.


Similar content being viewed by others
References
Moura FA, Fonseca Goulart MO. Chapter 7 - inflammatory bowel diseases: the crosslink between risk factors and antioxidant therapy. In: Gracia-Sancho J, Salvado J, eds. Maceio: Academic Press; 2017; 99–112.
Henao MP, Bewtra M, Osterman MT et al. Measurement of inflammatory bowel disease symptoms: reliability of an abbreviated approach to data collection. Inflamm Bowel Dis. 2015;21:2262–2271.
Kwon HJ, Dudley-Brown S, Williams M et al. Validation of a simple, patient directed, symptom based index for intestinal inflammation. J Clin Gastroenterol Treat. 2016;2:1–5.
Benitez JM, Meuwis MA, Reenaers C et al. Role of endoscopy, cross-sectional imaging and biomarkers in Crohn’s disease monitoring. Gut. 2013;62:1806–1816.
Bray C, Bell LN, Liang H et al. Erythrocyte sedimentation rate and c-reactive protein measurements and their relevance in clinical medicine. WMJ. 2016;115:317–321.
Hoekman DR, Diederen K, Koot BGP et al. Relationship of clinical symptoms with biomarkers of inflammation in pediatric inflammatory bowel disease. Eur J Pediatr. 2016;175:1335–1342.
Solem CA, Loftus EV, Tremaine WJ et al. Correlation of c-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712.
Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterol. 2011;140:1817–1826.
Wagatsuma K, Yokoyama Y, Nakase H. Role of biomarkers in the diagnosis and treatment of inflammatory bowel disease. Life. 2021;11:1375–1396.
Mihai C, Prelipcean CC, Dranga M et al. Correlations between inflammatory biomarkers and activity in inflammatory bowel diseases. Rev Chim. 2018;69:710–713.
Chang S, Malter L, Hudesman D. Disease monitoring in inflammatory bowel disease. World J Gastroenterol. 2015;21:11246–11259.
Fagerberg UL, Lööf L, Myrdal U et al. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;40:450–455.
Waugh N, Cummins E, Royle P et al. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17:1–212.
Sakurai T, Saruta M. Positioning and usefulness of biomarkers in inflammatory bowel disease. Digestion. 2023;104:30–41.
Boon GJ, Day AS, Mulder CJ et al. Are faecal markers good indicators of mucosal healing in inflammatory bowel disease? World J Gastroenterol. 2015;21:11469–11480.
Jha AK, Chaudhary M, Dayal VM et al. Optimal cut-off value of fecal calprotectin for the evaluation of ulcerative colitis: an unsolved issue? JGH Open. 2018;2:207–213.
Sipponen T, Haapamäki J, Savilahti E et al. Fecal calprotectin and S100A12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand J Gastroenterol. 2012;47:778–784.
Thia K, Faubion WA, Loftus EV et al. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis. 2011;17:105–111.
Lewis JD, Chuai S, Nessel L et al. Use of the non-invasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008;14:1660–1666.
Lewis JD. C-reactive protein: anti-placebo or predictor of response. Gastroenterol. 2005;129:1114–1116.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. New York: Springer; 2016.
R Core Team, R: A language and environment for statistical computing., R.F.f.S. Computing, Editor. 2021: Vienna, Austria.
Pinheiro J, Bates D, DebRoy S, Sarkar D. R Core Team., _nlme: Linear and Nonlinear Mixed Effects Models_. 2021.
Christensen RHB. _ordinal-Regression Models for Ordinal Data_. R Package version 2023: 12–4.
Colombel JF, Panaccione R, Bossuyt P et al. Effect of tight control management of Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2017;390:2779–2789.
Bakkaloglu OK, Eskazan T, Celik S et al. Can we predict mucosal remission in ulcerative colitis more precisely with a redefined cutoff level of C-reactive protein? Colorectal Dis. 2022;24:77–84.
Walsh A, Kormilitzin A, Hinds C et al. Defining faecal calprotectin thresholds as a surrogate for endoscopic and histological disease activity in ulcerative colitis—a prospective analysis. J Crohns Colitis. 2019;19:424–430.
Cartier A, Côté M, Lemieux I et al. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? The Am J Clinl Nutr. 2009;89:1307–1314.
Acknowledgments
The results published here are in whole based on data from the Study of a Prospective Adult Research Cohort with IBD (SPARC IBD). SPARC IBD is a component of the Crohn’s & Colitis Foundation’s IBD Plexus data exchange platform. SPARC IBD enrolls patients with an established or new diagnosis of IBD from sites throughout the United States and links data collected from the electronic health record and study specific case report forms. Patients also provide, stool, and biopsy samples at selected times during follow-up. The design and implementation of the SPARC IBD cohort have been previously described. SPARC-IBD investigators and affiliations: Kirk Russ5, Meena Bewtra6, James Lewis6, Raymond Cross7, Uni Wong7, Scott Snapper8, Josh Korzenik8, Shrinivas Bishu9, Rick Duerr10, Sumona Saha11, Freddy Caldera11, Laura Raffals12, Richa Shukla13, Themistocles Dassopoulos14, Matthew Bohm15, Poonam Beniwal-Patel16, David Hudesman17, Lauren Brook18, Joel Pekow19, Elizabeth Scoville20, Matthew Cioba21, Parakkal Deepak21, 5. University of Alabama, 6. University of Pennsylvania, 7. University of Maryland, 8. Brigham & Women's Hospital, 9. University of Michigan, 10. University of Pittsburgh, 11. University of Wisconsin, 12. Mayo Clinic, 13. Baylor College of Medicine, 14. Baylor Scott & White, 15. Indiana University, 16. Medical College of Wisconsin, 17. NYU Langone Medical Center, 18. University of Cincinnati, 19. University of Chicago, 20. Vanderbilt University, 21. Washington University, USA
Funding
Madeline Alizadeh—supported by the National Institutes of Diabetes and Digestive and Kidney Diseases, of the National Institutes of Health, under award number T32DK067872. The other authors have no other financial disclosers.
Author information
Authors and Affiliations
Consortia
Contributions
All authors have made significant contributions to this manuscript and have approved the final version to be submitted. KKM contributed toward study conception and design, interpretation of data, and drafting of article. MA contributed toward data acquisition and organization, interpretation of data, and drafting of article. AA contributed toward data acquisition and organization, interpretation of data, and revision of the article for important intellectual content. JG contributed toward interpretation of data and drafting of article. JW contributed toward study conception and design, and revision of the article for important intellectual content. RKC contributed toward acquisition of data, data interpretation, and revision of the article for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
Kiran K. Motwani, Madeline Alizadeh, Ameer Abutaleb, Jennifer Grossman, Jennifer Wellington, and Raymond K. Cross have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Motwani, K.K., Alizadeh, M., Abutaleb, A. et al. Correlation Between Serum and Fecal Biomarkers and Patient-Reported Outcomes in Patients with Crohn’s Disease and Ulcerative Colitis. Dig Dis Sci (2024). https://doi.org/10.1007/s10620-024-08421-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10620-024-08421-w