Abstract
Goals and Background
Ustekinumab (UST) is a monoclonal antibody inhibitor of IL-12/IL-23 approved for the treatment of Crohn’s disease (CD) and ulcerative colitis (UC). We conducted a meta-analysis to compare rates of adverse events (AEs) in randomized controlled trials (RCTs) of UST for all indications.
Study
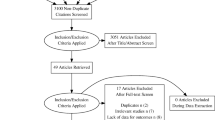
A systematic search was performed of MEDLINE, Embase, and PubMed databases through November 2019. Study inclusion included RCTs comparing UST to placebo or other biologics in patients aged 18 years or older with a diagnosis of an autoimmune condition.
Results
Thirty RCTs with 16,068 patients were included in our analysis. Nine thousand six hundred and twenty-six subjects were included in the UST vs placebo analysis. There was no significant difference in serious or mild/moderate AEs between UST and placebo with an OR of 0.83 (95% CI 0.66, 1.05) and 1.08 (95% CI 0.99, 1.18), respectively, over a median follow-up time of 16 weeks. In a sub-analysis of CD and UC trials, no difference in serious or mild/moderate AEs in UST versus placebo was seen.
Conclusions
UST was not associated with an increase in short-term risk of AEs.

Similar content being viewed by others
References
Judson MA, Baughman RP, Costabel U, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J. 2014;44:1296–1307. https://doi.org/10.1183/09031936.00000914.
Igarashi A, Kato T, Kato M, Song M, Nakagawa H, Japanese Ustekinumab Study Group. Efficacy and safety of ustekinumab in Japanese patients with moderate-to-severe plaque-type psoriasis: long-term results from a phase 2/3 clinical trial. J Dermatol. 2012;39:242–252. https://doi.org/10.1111/j.1346-8138.2011.01347.x.
Gottlieb A, Menter A, Mendelsohn A, et al. Ustekinumab, a human interleukin 12/23 monoclonal antibody, for psoriatic arthritis: randomised, double-blind, placebo-controlled, crossover trial. Lancet Lond Engl. 2009;373:633–640. https://doi.org/10.1016/S0140-6736(09)60140-9.
Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet Lond Engl. 2008;371:1665–1674. https://doi.org/10.1016/S0140-6736(08)60725-4.
Papp KA, Langley RG, Lebwohl M, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 52-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 2). Lancet Lond Engl. 2008;371:1675–1684. https://doi.org/10.1016/S0140-6736(08)60726-6.
Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318–1328. https://doi.org/10.1056/NEJMoa1503824.
McInnes IB, Kavanaugh A, Gottlieb AB, et al. Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial. Lancet Lond Engl. 2013;382:780–789. https://doi.org/10.1016/S0140-6736(13)60594-2.
Segal BM, Constantinescu CS, Raychaudhuri A, et al. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol. 2008;7:796–804. https://doi.org/10.1016/S1474-4422(08)70173-X.
Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73:990–999. https://doi.org/10.1136/annrheumdis-2013-204655.
Khattri S, Brunner PM, Garcet S, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. 2017;26:28–35. https://doi.org/10.1111/exd.13112.
Sandborn WJ, Gasink C, Gao L-L, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012;367:1519–1528. https://doi.org/10.1056/NEJMoa1203572.
Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375:1946–1960. https://doi.org/10.1056/NEJMoa1602773.
Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008;135:1130–1141. https://doi.org/10.1053/j.gastro.2008.07.014.
Blauvelt A, Reich K, Tsai T-F, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate-to-severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–69.e9. https://doi.org/10.1016/j.jaad.2016.08.008.
Griffiths CEM, Strober BE, van de Kerkhof P, et al. Comparison of ustekinumab and etanercept for moderate-to-severe psoriasis. N Engl J Med. 2010;362:118–128. https://doi.org/10.1056/NEJMoa0810652.
Gelfand JM, Shin DB, Alavi A, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U Trial). J Invest Dermatol. 2020;140:85–93.e2. https://doi.org/10.1016/j.jid.2019.07.679.
van Vollenhoven RF, Hahn BH, Tsokos GC, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet Lond Engl. 2018;392:1330–1339. https://doi.org/10.1016/S0140-6736(18)32167-6.
Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381:1201–1214. https://doi.org/10.1056/NEJMoa1900750.
Saeki H, Kabashima K, Tokura Y, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol. 2017;177:419–427. https://doi.org/10.1111/bjd.15493.
Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet Lond Engl. 2018;392:650–661. https://doi.org/10.1016/S0140-6736(18)31713-6.
Deodhar A, Gensler LS, Sieper J, et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of Ustekinumab in axial spondyloarthritis. Arthritis Rheumatol Hoboken NJ. 2019;71:258–270. https://doi.org/10.1002/art.40728.
Smolen JS, Agarwal SK, Ilivanova E, et al. A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann Rheum Dis. 2017;76:831–839. https://doi.org/10.1136/annrheumdis-2016-209831.
Paul C, Griffiths CEM, van de Kerkhof PCM, et al. Ixekizumab provides superior efficacy compared with ustekinumab over 52 weeks of treatment: results from IXORA-S, a phase 3 study. J Am Acad Dermatol. 2019;80:70–79.e3. https://doi.org/10.1016/j.jaad.2018.06.039.
Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week clarity results). Dermatol Ther. 2018;8:571–579. https://doi.org/10.1007/s13555-018-0265-y.
Deodhar A, Gottlieb AB, Boehncke W-H, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Lond Engl. 2018;391:2213–2224. https://doi.org/10.1016/S0140-6736(18)30952-8.
Frucht DM, Holland SM. Defective monocyte costimulation for IFN-gamma production in familial disseminated Mycobacterium avium complex infection: abnormal IL-12 regulation. J Immunol Baltim Md 1950. 1996;157:411–416.
Godinez I, Keestra AM, Spees A, Bäumler AJ. The IL-23 axis in Salmonella gastroenteritis. Cell Microbiol. 2011;13:1639–1647. https://doi.org/10.1111/j.1462-5822.2011.01637.x.
Awoniyi M, Miller SI, Wilson CB, Hajjar AM, Smith KD. Homeostatic regulation of Salmonella-induced mucosal inflammation and injury by IL-23. PLoS ONE. 2012;7:e37311. https://doi.org/10.1371/journal.pone.0037311.
Yan J, Smyth MJ, Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. 2017;. https://doi.org/10.1101/cshperspect.a028530.
Schurich A, Raine C, Morris V, Ciurtin C. The role of IL-12/23 in T cell-related chronic inflammation: implications of immunodeficiency and therapeutic blockade. Rheumatol Oxf Engl. 2017. https://doi.org/10.1093/rheumatology/kex186.
Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 (Updated on March 2011) 2011.
Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn’s disease. Gastroenterol Clin N Am. 2017;46:603–626. https://doi.org/10.1016/j.gtc.2017.05.013.
Macaluso FS, Maida M, Ventimiglia M, Cottone M, Orlando A. Effectiveness and safety of Ustekinumab for the treatment of Crohn’s disease in real-life experiences: a meta-analysis of observational studies. Expert Opin Biol Ther. 2020;20:193–203. https://doi.org/10.1080/14712598.2020.1707800.
Funding
There was no funding source for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Hudesman was the consultant for Pfizer, Takeda, Janssen Biotech, Abbvie, and Salix. Research support was from Pfizer. Shannon Chang was the consultant for Takeda, Pfizer, and Oshi Health. Lisa Malter was the consultant for Takeda, Pfizer, Abbvie, Prometheus, Janssen, Merck, UCB, and Gilead. The remaining authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rolston, V.S., Kimmel, J., Popov, V. et al. Ustekinumab Does Not Increase Risk of Adverse Events: A Meta-Analysis of Randomized Controlled Trials. Dig Dis Sci 66, 1631–1638 (2021). https://doi.org/10.1007/s10620-020-06344-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06344-w