Abstract
Background
Crohn disease (CD) is an inflammatory bowel disease which occurs especially in developed countries of Western Europe and North America. The aim of the study was to compare the safety profile of biologic drugs in patients with CD.
Methodology
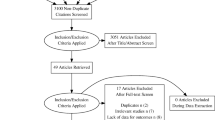
A systematic literature search was performed using PubMed, Embase, and CENTRAL databases, until April 27, 2016. We included randomized controlled trials (RCTs) that compared the safety of biologic drugs (infliximab, adalimumab, vedolizumab, certolizumab pegol, and ustekinumab) with one another or with placebo in patients with CD. The network meta-analysis (NMA) was conducted for an induction phase (6–10 weeks) and maintenance phase (52–56 weeks) with a Bayesian hierarchical random effects model in the ADDIS® software. The PROSPERO registration number was CRD42016032606.
Results
Ten RCTs were included in the systematic review with NMA. In the case of the induction phase, the NMA could be conducted for the assessment of the relative safety profile of adalimumab, vedolizumab, certolizumab pegol, and ustekinumab, and in the case of the maintenance phase—of infliximab, adalimumab, and vedolizumab. There were no significant differences in the rate of adverse events in patients treated with biologics. Statistical analysis revealed that vedolizumab had the greatest probability of being the safest treatment in most endpoints in the induction phase and adalimumab—in the maintenance phase.
Conclusions
No significant differences between the biologics in the relative safety profile analysis were observed. Further studies are needed to confirm our findings, including head-to-head comparisons between the analyzed biologics.
Similar content being viewed by others
References
Lin L, Liu X, Wang D, Zheng C. Efficacy and safety of antiintegrin antibody for inflammatory bowel disease: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(10):e556.
Carter MJ, Lobo AJ, Travis SPL. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004;53(Suppl. 5):V1–V16.
Moja L, Danese S, Fiorino G, Del Giovane C, Bonovas S. Systematic review with network meta-analysis: comparative efficacy and safety of budesonide and mesalazine (mesalamine) for Crohn’s disease. Aliment Pharmacol Ther 2015;41(11):1055–65.
Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46–54.
Johnson & Johnson. Available from: https://doi.org/www.investor.jnj.com/releasedetail.cfm?ReleaseID=944699 (May 10, 2016)
Brignardello-Petersen R, Rochwerg B, Guyatt GH. What is a network meta-analysis and how can we use it to inform clinical practice? Pol Arch Med Wewn 2014;124(12):659–60.
Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159(2):130–7.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analysis of health care interventions: checklist and explanations. Ann Intern Med 2015;162(11):777–84.
Jansen JP, Trikalinos T, Cappelleri JC, Daw J, Andes S, Eldessouki R, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014; 17 (2): 157–73.
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011.
Mocko P, Kawalec P, Pilc A. Analysis of the safety profile of biologic drugs in the therapy of Crohn’s disease: a systematic review and network meta-analysis. PROSPERO 2016 CRD42016032606. Available from: https://doi.org/www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016032606.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60.
van Valkenhoef G, Tervonen T, Zwinkels T, de Brock B, Hillege H. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst 2013;55:459–75.
Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7(4):434–55.
Jonas DE, Wilkins TM, Bangdiwala S, Bann CM, Morgan LC, Thaler KJ, et al. Findings of bayesian mixed treatment comparison meta-Analyses: comparison and exploration using real-World trial data and simulation. AHRQ 2013 No.:13-EHC039-EF.
Ades AE, Welton N, Lu G. Introduction to Mixed Treatment Comparisons. Available from: https://doi.org/www.bristol.ac.uk/social-community-medicine/media/mpes/intro-to-mtc.pdf.
Wu HY, Huang JW, Lin HJ, Liao WC, Peng YS, Hung KY, et al. Comparative effectiveness of renin-angiotensin system blockers and other antihypertensive drugs in patients with diabetes: systematic review and Bayesian network meta-analysis. BMJ 2013;347:f6008.
Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002;359:1541–9.
Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004;350:876–85.
Regueiro M, Schraut W, Baidoo L, Kip KE, Sepulveda AR, Pesci M, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
Watanabe M, Hibi T, Lomax KG, Paulson SK, Chao J, Alam MS, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis 2012;6:160–73.
Colombel J-F, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 2007;132:52–65.
Sandborn WJ, Hanauer SB, Rutgeerts P, Fedorak RN, Lukas M, MacIntosh DG, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut 2007;56:1232–9.
Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013;369(8):711–21.
Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factorantagonist treatment failed. Gastroenterology 2014; 147 (3):618–27.
Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, et al. Certolizumab pegol for active Crohn’s disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol 2011;9:670–8.
Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012;367(16):1519–28.
Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn’s disease: a network meta-analysis. Gastroenterology 2015; 148(2):344–54.
Singh JA, Wells GA, Christensen R, Tanjong Ghogomu E, Maxwell L, Macdonald JK, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview (Review). Cochrane Database Syst Rev 20112: CD008794.
Singh S, Garg SK, Pardi DS, Wang Z, Murad MH, Loftus Jr. EV. Comparative efficacy of biologic therapy in biologic-naïve patients with Crohn disease: a systematic review and network meta-analysis. Mayo Clin Proc 2014;89 (12):1621–35.
Author information
Authors and Affiliations
Corresponding author
Supplementary data
Rights and permissions
About this article
Cite this article
Moćko, P., Kawalec, P. & Pilc, A. Safety profile of biologic drugs in the therapy of Crohn disease: A systematic review and network meta-analysis. Pharmacol. Rep 68, 1237–1243 (2016). https://doi.org/10.1016/j.pharep.2016.07.013
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2016.07.013