Abstract
Background
Irritable bowel syndrome (IBS) occurs in up to 33% of Gulf War (GW) Veterans. Alterations in gut microflora including small intestinal bacterial overgrowth (SIBO) during deployment may play a role in development of IBS. Rifaximin is a minimally absorbed antibiotic speculated to improve IBS symptoms, in part, by restoring normal gut microflora. The aim of this study was to compare rifaximin to placebo on IBS symptoms and quality of life (QOL) in GW Veterans with IBS without constipation.
Methods
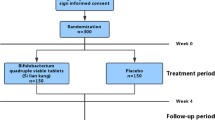
A double-blind, placebo-controlled study was performed. One hundred and twenty-two GW Veterans with IBS (Rome III) from our database and referral to gastroenterology and internal medicine clinics were screened. After a 2-week run-in period, 50 patients were randomized (1:1) to receive either rifaximin 550 gm or placebo twice daily for 2 weeks in a double-blind study. Patients were advised not to change their diet or medications during the study. The symptoms assessed were: (1) stool frequency, (2) stool consistency (Bristol stool scale, 1–7, very hard to watery), (3) urgency (1 = yes/0 = no daily for 7 days), (4) severity of abdominal pain (0–4, none to severe), (5) severity of bloating (1–4, none to severe), and (6) global improvement scale (1–7, substantially worse to substantially improved). These were recorded for 7 consecutive days and then averaged across the 7 days, to generate a continuous variable. The symptom data were compared after 2 weeks of treatment. QOL was assessed using IBS-QOL. The lactulose hydrogen breath test (LHBT) was performed at baseline and after 2 weeks of treatment.
Results
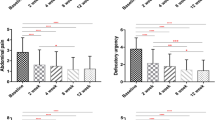
Fifty Veterans were randomized to receive treatment; 3 withdrew and 3 were lost to follow-up. Data were analyzed from 44 patients (38 men, 6 women, median age 52, range 33–77 years). Rifaximin was not associated with significant improvement in global symptoms, abdominal pain, bloating, stool urgency, frequency, or consistency (all P ≥ 0.25) or QOL (all P ≥ 0.26). Normalization of SIBO by LHBT was not different between rifaximin- and placebo-treated Veterans (7 vs. 22%, P = 0. 54).
Conclusion
Rifaximin was not effective in improving IBS symptoms and QOL in GW Veterans with non-constipated IBS.



Similar content being viewed by others
References
Chronic Multisymptom Illness in Gulf War Veterans. Case Definitions Reexamined. Institute of Medicine: National Academy of Sciences, National Academies Press; 2014.
Gulf War and Health. Update of health effects of serving in the Gulf War. Institute of Medicine: National Academy of Sciences, National Academies Press; 2016.
Steele L. Prevalence and patterns of Gulf War illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol. 2000;152:992–1002.
Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491.
Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. 2015;313:949–958.
Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome–a meta-analysis. Am J Gastroenterol. 2006;101:1894–1899. quiz 942.
Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–3506.
Hyams KC, Bourgeois AL, Merrell BR, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428.
Porter CK, Choi D, Cash B, et al. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC Gastroenterol. 2013;13:46.
Khoshini R, Dai SC, Lezcano S, Pimentel M. A systematic review of diagnostic tests for small intestinal bacterial overgrowth. Dig Dis Sci. 2008;53:1443–1454.
Ford AC, Spiegel BM, Talley NJ, Moayyedi P. Small intestinal bacterial overgrowth in irritable bowel syndrome: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2009;7:1279–1286.
Huang DB, DuPont HL. Rifaximin–a novel antimicrobial for enteric infections. J Infect. 2005;50:97–106.
Meyrat P, Safroneeva E, Schoepfer AM. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment Pharmacol Ther. 2012;36:1084–1093.
Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32.
Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333.
Talley NJ, Phillips SF, Melton J 3rd, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674.
Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400–411.
Derogatis L. Brief Symptom Inventory-18 (BSI-18): Administration, Scoring, and Procedure Manual. Ed 3 ed. Minneapolis, MN: National Computer Systems; 2000.
Bratten JR, Spanier J, Jones MP. Lactulose breath testing does not discriminate patients with irritable bowel syndrome from healthy controls. Am J Gastroenterol. 2008;103:958–963.
Levitt MD, Furne JK, Kuskowski M, Ruddy J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol. 2006;4:123–129.
Kassinen A, Krogius-Kurikka L, Makivuokko H, et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33.
Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563.
Acosta A, Camilleri M, Shin A, et al. Effects of Rifaximin on Transit, Permeability, Fecal Microbiome, and Organic Acid Excretion in Irritable Bowel Syndrome. Clin Transl Gastroenterol. 2016;7:e173.
Menees SB, Maneerattannaporn M, Kim HM, Chey WD. The efficacy and safety of rifaximin for the irritable bowel syndrome: a systematic review and meta-analysis. Am J Gastroenterol. 2012;107:28–35. quiz 6.
Rezaie A, Buresi M, Lembo A, et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American consensus. Am J Gastroenterol. 2017;112:775–784.
Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419.
Shah ED, Basseri RJ, Chong K, Pimentel M. Abnormal breath testing in IBS: a meta-analysis. Dig Dis Sci. 2010;55:2441–2449.
Gasbarrini A, Corazza GR, Gasbarrini G, et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29:1–49.
Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut. 2011;60:334–340.
Rezaie A, Pimentel M, Rao SS. How to test and treat small intestinal bacterial overgrowth: an evidence-based approach. Curr Gastroenterol Rep. 2016;18:8.
Saad RJ, Chey WD. Breath testing for small intestinal bacterial overgrowth: maximizing test accuracy. Clin Gastroenterol Hepatol. 2014;12:1964–1972. quiz e119-20.
Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017;312:G52–G62.
Tuteja AK. Deployment-associated functional gastrointestinal disorders: do we know the etiology? Dig Dis Sci. 2011;56:3109–3111.
Acknowledgments
The authors thank Dr. Keith G. Tolman, M.D. for assistance in editing the initial proposal and periodic consultation. The study drug and placebo tablets were provided by Salix Pharmaceuticals.
Funding
AKT received grant support from the Department of Veterans Affairs and the Department of Defense (W81XWH-10-1-0593, W81XWH-15-1-0636). GNV received support from the Department of Veterans Affairs (1I01CX001477-01) and the National Institute of Diabetes and Digestive and Kidney Diseases (DK099052).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AKT has received funding from Shire and Synergy Pharmaceuticals. NJT Grant/Research Support: Rome Foundation; Abbott Pharmaceuticals; Datapharm; Pfizer; Salix [Irritable bowel syndrome]; Prometheus Laboratories Inc [Irritable bowel syndrome (IBS Diagnostic)]; Janssen [Constipation]. Consultant/Advisory Boards: Adelphi Values [Functional dyspepsia (patient-reported outcome measures)]; (Budesonide)]; GI therapies [Chronic constipation (Rhythm IC)]; Sax Institute; Allergens PLC; Napo Pharmaceutical; Outpost Medicine; Samsung Bioepis; Yuhan [IBS]; Synergy [IBS]; Theravance [Gastroparesis]; Patent Holder: Biomarkers of irritable bowel syndrome [Irritable bowel syndrome] Licensing Questionnaires [Mayo Clinic Talley Bowel Disease Questionnaire—Mayo Dysphagia Questionnaire]; Nestec European Patent [Application No. 12735358.9]; Singapore “Provisional” Patent [NTU Ref: TD/129/17 “Microbiota Modulation Of BDNF Tissue Repair Pathway”].
Additional information
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs or the Department of Defense.
Rights and permissions
About this article
Cite this article
Tuteja, A.K., Talley, N.J., Stoddard, G.J. et al. Double-Blind Placebo-Controlled Study of Rifaximin and Lactulose Hydrogen Breath Test in Gulf War Veterans with Irritable Bowel Syndrome. Dig Dis Sci 64, 838–845 (2019). https://doi.org/10.1007/s10620-018-5344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5344-5