Abstract
Objectives
Current irritable bowel syndrome (IBS) treatments have limited efficacy and probiotics like Bacillus clausii (B. clausii) were found to be effective in the management of several gastrointestinal disorders. This phase III trial assessed the efficacy and safety of adding B. clausii (four strains: O/C, N/R, SIN, T), versus placebo, to conventional treatment of pediatric IBS in Mexico.
Methods
Patients aged 6–17 years 11 months with IBS (Rome IV) for at least 2 months were randomized to receive either B. clausii (oral suspension, total dose 4 billion spores/day) or placebo once daily for 8 weeks. All patients also received conventional treatment. The primary endpoint was the difference in the proportion of patients with clinical improvements at Week 8 (Global Assessment Questions [GAQ]). Secondary endpoints included responders by Subject’s Global Assessment of Relief for Children with IBS (SGARC); number/consistency of stools; abdominal distention/bloating; abdominal pain/intensity; and IBS behavior.
Results
73.6% (95% confidence interval [CI] 67.3–80.0; B. clausii n = 129) and 78.5% (95% CI 72.5–84.4; placebo n = 130) of patients had symptom improvement (p = 0.8182). For Week 8 SGARC, 19.2% (B. clausii) and 20.9% (placebo) reported complete symptom relief. Stool evaluations, bloating, abdominal pain/intensity, and IBS behavior were similar between groups. Both treatments were well tolerated.
Conclusion
No significant differences in efficacy between B. clausii and placebo were demonstrated in addition to conventional treatment. The sample size calculation was based on an expected placebo/conventional treatment response of 30–40%. However, the actual treatment response observed was 80% and, thus, a study with larger population would be warranted. In addition, this study was conducted during the COVID-19 pandemic, when such controlled social conditions may have resulted in better diet, greater family stability, less psychological stress, and lower risk of infections exacerbating IBS, thereby improving symptoms in both groups.
EudraCT number
2018-004519-31.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Probiotics targeting the gut microbiota have beneficial effects in functional gastrointestinal disorders in children and adults. |
This phase III trial in children with IBS demonstrated that treatment with Bacillus clausii strains as add-on to conventional treatment was well tolerated; although no additional benefit was seen in relieving symptoms over 8 weeks compared with placebo with conventional treatment, significant improvements in abdominal pain episodes (Week 4) and significant decreases in abdominal bloating in patients with IBS with constipation occurred with B. clausii versus placebo. |
Placebo responses were higher than expected; as the study was conducted under controlled social conditions during the COVID-19 pandemic, improved conditions (e.g., better diet, greater family stability, reduced school-induced stress, lower risk of infections) may have improved IBS symptoms in both groups. |
1 Introduction
The estimated prevalence of irritable bowel syndrome (IBS) in children ranges from 2.8 to 22.6% (Rome III or IV criteria), reflecting geography and study types [1]. IBS is linked to reduction in quality of life [1], stress, anxiety, depression, and emotional problems [2], and results in absenteeism [3]. Thus, IBS is considered both a health and a socioeconomic burden.
Curative treatment for IBS is currently not available and current management strategies vary. Conventional treatment includes diet adjustment and confirmation [4], explanation of pain experience and reassurance, symptomatic treatments such as laxatives for constipation and possibly analgesics [5] or antispasmodics [6], with limited efficacy. Pharmacological therapy may reduce discomfort caused by diarrhea [7] or constipation [8]. Antidepressants and psychological therapies are also used in IBS [9]; although there are concerns with using antidepressants in children.
Gut microbiota dysbiosis is increasingly considered as a vital factor in the etiopathogenesis of IBS; thus, gut microbiota are a potential therapeutic target [10]. A commercial probiotic (Enterogermina®) comprises four strains of Bacillus clausii (B. clausii; O/C, N/R, SIN, T) [11]. B. clausii has demonstrated beneficial effects in adults, such as in ameliorating bacterial overgrowth [12], acute diarrhea [13, 14], diarrhea associated with Clostridium difficile [15], and as an adjuvant in treating Helicobacter pylori [16, 17]. Importantly, benefits of B. clausii have also been demonstrated in children, such as in antibiotic-associated diarrhea [18, 19], diarrhea [13, 14, 20,21,22,23,24,25], and rotavirus-associated diarrhea [26, 27].
Data from a small pilot study in a third-level hospital in Mexico demonstrated that B. clausii treatment significantly improved several symptoms in children with IBS [28]. Consequently, the phase III trial reported herein investigated the efficacy and safety of B. clausii plus conventional treatment, compared with placebo plus conventional treatment, in children with IBS in Mexico.
2 Methods
2.1 Study Design and Patients
BaclauSII (EudraCT number: 2018-004519-31) was a phase III, multicenter, randomized, placebo-controlled, double-blind, parallel clinical trial conducted at 15 study sites in Mexico. There were three study phases, a 2-week run-in (in which ongoing IBS treatment was continued), an 8-week treatment period, and an 8-week follow-up period (no treatment) (Supplementary Fig. 1, see electronic supplementary material [ESM]), which are in keeping with published recommendations [29, 30].
Male or female patients aged 6–17 years and 11 months with IBS (Rome IV criteria [31]) were eligible. IBS diagnostic criteria had to be met for at least 2 months before diagnosis, and had to include all of the following: abdominal pain for ≥ 4 days per month (associated with > 1 of the following: related to a bowel movement; a change in stool frequency; a change in stool appearance); in children with constipation, the pain did not resolve with resolution of the constipation (i.e., not functional constipation); after appropriate evaluation, the symptoms cannot be fully explained by another medical condition.
Key exclusion criteria included treatment with antibiotics or probiotics within 2 months prior to screening; growth failure or malnutrition; previous abdominal surgery; known gastrointestinal comorbidity (e.g., inflammatory bowel disease, celiac disease, H. pylori infection); lactose intolerance, without a diet eliminating lactose; history of bleeding from the low digestive tract in the 2 years prior or had abnormal endoscopic or histological studies; history of significant infections or inflammatory processes during pre-enrollment; any patient not suitable for participation, regardless of reason, as judged by the Investigator (including medical or clinical conditions, or patients potentially at risk of noncompliance to study procedures).
2.2 Ethics
The protocol was approved by Independent Ethics Committees and Research Committees. The study was conducted in accordance with all applicable laws, rules, and regulations, and with the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice. Parents/legal guardians provided written, informed consent on behalf of their child at the time of enrollment. An informed assent form was signed by the patient, if applicable (age of assent was determined by the Independent Ethics Committees and Research Committees).
2.3 Treatments
Patients were centrally randomized 1:1 to treatment with either B. clausii or placebo using an interactive web response system.
Bacillus clausii preparations consisted of spores at 2 × 109 colony forming units (CFU) in 5 mL of a ready-to-drink, oral suspension (total dose 4 × 109 CFU/day), which was an odorless, colorless, and insipid liquid. Placebo preparations were developed to exactly mimic the physical characteristics of the B. clausii preparations. Patients, investigators, and other personnel were blinded to treatment; kits were only distinguishable by the randomization number. Treatment unblinding was only conducted in response to an adverse event (AE) requiring additional care.
Patients swallowed the contents of two appropriate 5-mL vials once daily every morning before a meal for 8 weeks. Both groups had access to the usual standard of care as per general recommendations (see ESM). On day 1 (study start) and day 28 (±3 days), a sufficient number of vials of either B. clausii or placebo were dispensed to each patient/parent/legal guardian to cover the time period until the next visit. Full instructions were given to patients/parents/legal guardians for study and conventional treatments (including storage conditions for study treatments, i.e., at room temperature [not more than 30 °C] away from direct light and moisture). Compliance with storage conditions was not monitored. There is evidence that B. clausii can survive in liquid media and even grow at temperatures of 20–45 °C [32]. Each patient had a diary for data recording by the patient/parent/legal guardian. These diaries were to capture the frequency and severity of their IBS symptoms, to meet recommendations that IBS clinical trials should account for fluctuations in symptoms and the potential for wide variations in bowel habits [29, 33, 34]. Evaluation visits were conducted at Weeks 4 and 8 (end of treatment). The follow-up period (no treatment) was for 8 weeks and patients were evaluated at the end of this period (Week 16) (Supplementary Fig. 1, see ESM).
2.4 Study Assessments
Treatment intake was recorded daily in the diary. The primary endpoint was the difference in the proportion of treatment responders between groups after 8 weeks of treatment. Response rate at Week 8 was defined as patients with clinical improvement of symptoms in the following Global Assessment Questions (GAQ): “How well did the medication relieve your symptoms? (satisfaction with treatment; excellent, good, fair, poor, failed)” rated as excellent or good and “Overall, how do you feel your problem is? (symptom relief; worse, same, better)” rated as better. The GAQ assesses the patient’s overall relief of symptoms (wellbeing, symptoms of stomach discomfort, pain, and altered bowel habits) [34,35,36,37]. Several secondary endpoints were evaluated (i.e., response rate at Week 4; proportion of responders at Weeks 4 and 8; number of stools/day; stool consistency; abdominal distention/bloating; abdominal pain episodes by day; pain intensity; and IBS behavior). Response rate at Week 4 was assessed by GAQ. Proportion of treatment responders at Weeks 4 and 8 was evaluated by the Subject’s Global Assessment of Relief for Children with IBS (SGARC) [38,39,40]; treatment response was defined as 0 = complete relief or 1 = considerable relief. At Weeks 4, 8, and 16, records were made in patient diaries for number of stools by day; consistency of stools (Bristol Stool Form Scale); abdominal distention/bloating (3-point scale); number of abdominal pain episodes by day; pain intensity (Face Pain Scale–Revised); and IBS behavior (Behavior Scale). These secondary endpoints are considered to be reliable measurements to evaluate the clinically important signs and symptoms associated with IBS [29, 33].
Safety was assessed by AE reporting in the diaries.
2.5 Data Analyses
Sample size was calculated for the primary endpoint. Based on certain assumptions (see ESM), 105 patients per group would be necessary to observe a success rate of at least 65% in the B. clausii arm (a 20% difference between groups), with 90% power and 5% significance level (one-sided). Considering a drop-out rate of ~ 20%, 260 randomized patients (130/group) were needed.
Demographics and baseline characteristics were summarized by using descriptive statistics (including n, %, mean and standard deviation [SD]) for each treatment group.
Treatment compliance (%) was defined as (administered doses/planned doses) × 100 and was summarized descriptively. A patient was considered treatment compliant if treatment intake was ≥ 80%. Treatment accountability (%) was defined as (used returned vials/planned doses) × 100.
The primary endpoint was evaluated by the Chi-square test or Fisher Exact test; 95% confidence intervals (CIs) were computed for the proportions in each treatment group and for the difference in the proportions between groups. The main analysis was conducted on the intent-to-treat (ITT) population with missing data considered as non-responders. A sensitivity analysis was repeated (no replacement of missing data) for the ITT population, and a supportive analysis was performed for the per protocol (PP) population.
Analyses of secondary endpoints were performed for the ITT population. In addition to descriptive statistics, several secondary endpoints were compared between treatment groups using the Chi-square test or Fisher Exact test (if the assumption of the Chi-square test was not verified). Percentage of bloating days and mean number of abdominal pain episodes were evaluated using The Mann-Whitney U test or Student’s t-test for independent samples.
In a subgroup analysis, the primary endpoint was analyzed by concomitant medication use and by IBS type. In an exploratory analysis, secondary endpoints were analyzed by IBS type.
AE incidences were summarized descriptively.
3 Results
3.1 Disposition and Baseline Characteristics
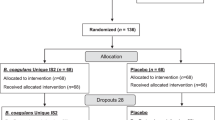
Overall, 311 patients were screened, and 259 (B. clausii n = 129; placebo n = 130) were randomized and treated (ITT population) (Fig. 1). In total, 253 patients (97.7%) (B. clausii n = 124, placebo n = 129) completed the study (Fig. 1). Reasons for discontinuation were withdrawal of consent (B. clausii n = 3); protocol violation (1 patient/group); and ‘other’ reasons (B. clausii n = 1).
Baseline demographic and clinical characteristics were generally well balanced between groups (Table 1). Of the laboratory parameters evaluated at Week −2 (see ESM), only one patient (0.8%) per group had an abnormal result with clinical significance for parasite examination seriate. IBS medication was taken before study start by 24% (31/129) and 26.2% (34/130) of patients in the B. clausii and placebo groups, respectively; the most frequent of which was in the alimentary tract and metabolism drug class (21.7% [28/129] vs 24.6% [32/130]). Within this class, drugs for functional gastrointestinal disorders were taken by 13.2% (17/129) and 13.8% (18/130) of patients, and drugs for constipation by 7.8% (10/129) and 6.2% (8/130) of patients in the B. clausii and placebo groups, respectively. Patients also took concomitant medication during the study (Table 1), the most common of which were drugs in the alimentary tract and metabolism drug class. Of these patients, 18.6% (24/129) and 25.4% (33/130) of patients in the B. clausii and placebo groups, respectively, took antispasmodics during the study period.
3.2 Treatment
In the safety population (patients receiving at least one dose of the appropriate formulation; B. clausii n = 129, placebo n = 130), mean (SD) of treatment exposure was 56.64 (6.23) and 57.67 (3.71) days for B. clausii and placebo, respectively. Treatment compliance was assessed in this population, and there was a total of 22 missing values related to product use (B. clausii 12 values missing, placebo 10 missing values). Thus, treatment compliance of > 80%, from diary cards, was seen in 81/117 (69.2%; B. clausii) and 77/120 (64.2%; placebo) of patients. In contrast, mean (SD) treatment accountability, from returns of used vials, was 95.60% (13.8) and 98.05% (8.48) for B. clausii and placebo, respectively. The difference between treatment compliance and treatment accountability was due to a discrepancy between the diary card information registered by the patients/caregivers compared with the treatment accountability measured by counting the actual vials consumed.
The PP population included 146 patients (B. clausii n = 75, placebo n = 71). Compliance was the key reason for exclusion from the PP population (B. clausii 45/129 [34.9%]; placebo 52/130 [40.0%]). Data from the PP population were only analyzed for the supportive analysis of the primary endpoint.
3.3 Proportion of Patients with Clinical Improvements at Week 8
For the primary endpoint (ITT analysis), 73.6% (95% CI 67.3–80.0) of patients had clinical improvement of symptoms with B. clausii versus 78.5% (95% CI 72.5–84.4) with placebo; p = 0.8182 (Table 2). The percentage of patients reporting excellent or good satisfaction with treatment was similar between groups (80.0% B. clausii; 80.6% placebo) (Supplementary Fig. 2, see ESM). For B. clausii, 28.8% of patients rated treatment as excellent in relieving symptoms, and 51.2% as good; whilst, with placebo, 33.3% and 47.3% of patients rated the treatment excellent and good, respectively. Considering overall symptom relief, 82.4% (B. clausii) and 90.7% (placebo) of patients felt better (Supplementary Fig. 2, see ESM).
Results of the sensitivity analyses were consistent with the primary analyses, and there were no significant differences between the groups (Table 2). Supportive analyses of the primary endpoint (PP population) showed that 80.0% (95% CI 72.4–87.6) of patients had clinical improvement of symptoms with B. clausii versus 78.9% (95% CI 70.9–86.8) of patients in the placebo group; p = 0.4332. The difference in proportions between groups was 1.1% (95% CI − 9.9 to 12.1). The percentage of patients reporting an excellent or good treatment satisfaction was similar between groups (33.3% B. clausii; 31.0% placebo).
3.4 Key Secondary Efficacy Endpoints
As the primary endpoint was not statistically significant, inferential analyses were not performed for secondary endpoints. Hence, p-values are shown for descriptive purposes only.
For the response rate at Week 4, 72.4% (92/129 B. clausii) and 76.7% (99/130 placebo) of patients showed clinical improvement in symptoms; p = 0.7855. For the clinical improvement of symptoms, assessed with SGARC at Week 8, 19.2% (24/126 B. clausii) and 20.9% (27/129 placebo) reported complete relief of symptoms (Supplementary Fig. 3, see ESM). Most patients reported considerable relief (56.8%, B. clausii; 64.3%, placebo). Overall, 76.0% of patients had a positive response to B. clausii, and 85.3% to placebo (p = 0.9694).
Results of stool evaluations and bloating were similar between groups with no significant differences (Supplementary Figs. 4–6, see ESM).
At Week 4, the median number of abdominal pain episodes (mean by day) was higher with placebo (0.55) versus B. clausii (0.47) (p = 0.0302). At Weeks 8 and 16, the median number of episodes was similar between groups (Supplementary Fig. 7, see ESM).
Percentage of abdominal pain episodes with a given pain intensity are shown in Supplementary Fig. 8 (see ESM). The median percentage with pain intensity 2 at Week 4 was 56.4% (B. clausii) and 69.2% (placebo; p = 0.0285). At Weeks 8 and 16, pain intensity 2 was the most frequently experienced pain intensity level in both groups. As pain intensity increased, the median percentage decreased at all timepoints (Supplementary Fig. 8, see ESM).
The categorical assessment of IBS behavior was similar between groups, with no significant differences at any timepoint. For all categories, most patients in both groups throughout the study considered that there were no disturbances in daily activities (Supplementary Fig. 9, see ESM).
3.5 Subgroup Analyses
For the subgroup analysis of the primary efficacy endpoint according to concomitant medication class (data not shown) and IBS type (Supplementary Table 1, see ESM), there were no statistically significant differences between the groups.
3.6 Exploratory Analyses
For the exploratory analyses of secondary endpoints, only three comparisons were statistically significant between the groups.
For abdominal distention/bloating by IBS type, the overall results were similar between groups and most patients reported feeling ‘better’ throughout the study. At Week 8, 70.4% of patients with IBS with diarrhea (IBS-D) in the B. clausii group rated their abdominal distention/bloating as ‘better’, which was significantly lower (p = 0.0432) than the placebo group (88.9%) (Supplementary Fig. 10, see ESM).
For patients with IBS with constipation (IBS-C), at Weeks 4 and 16 the median percentage of days with bloating was significantly lower in patients receiving B. clausii versus those receiving placebo: 8.1 versus 18.4 (Week 4; p = 0.0448) and 3.6 versus 9.5 (Week 16; p = 0.0224) (Supplementary Fig. 11, see ESM). No differences in bloating were seen at Week 8 in patients with IBS-C, or in patients with IBS-D or mixed IBS (IBS-M) at any timepoint (Supplementary Fig. 11, see ESM).
3.7 Safety and Tolerability
Overall, 52.7% (68/129, B. clausii) and 50.8% (66/130, placebo) of patients had at least one AE (Table 3). One serious AE was reported in the B. clausii group, comprising a case of febrile multisystem inflammatory syndrome of moderate severity lasting 2 days, which was not related to B. clausii and did not require medical intervention. The cause of this inflammatory syndrome was unknown, and COVID-19 testing was not performed in this study.
Overall, 44.2% (57/129, B. clausii) and 35.4% (46/130, placebo) of patients had at least one treatment-emergent AE (TEAE) (Table 3). Nine TEAEs in seven patients in the B. clausii group (2 patients discontinued treatment due to rhinopharyngitis [n = 1], and testing positive for pathogen parasites) and six TEAEs in five patients in the placebo group were treatment related. Incidences of TEAEs were similar between the groups (Table 3). The most frequently reported TEAEs by system organ class were upper respiratory infections (influenza was the most frequent in both groups), and nervous system disorders (headache was the most frequent in both groups). Vital signs (systolic blood pressure, diastolic blood pressure, heart rate, temperature) were similar between both groups throughout the study (data not shown).
4 Discussion
IBS symptom relief in children was found to be very high in both B. clausii and placebo groups, which exceeded expectations. There were no significant differences between groups for proportion of patients with clinical improvements at Week 8 or any of the key secondary endpoints. The AE profile was similar between groups.
More IBS treatments are needed to support conventional therapies [4,5,6,7,8,9]. In a meta-analysis of 20 IBS randomized clinical trials (RCTs), probiotics improved global IBS symptoms versus placebo (pooled relative risk 0.77, 95% CI 0.62–0.94) [41]. Probiotics significantly reduced abdominal pain (mean difference −1.15, 95% CI −2.05 to −0.24) in an analysis of nine RCTs in children with IBS [40]. Thus, gut microbiota remain as a good IBS therapeutic target [1, 10, 42].
Enterogermina® (four B. clausii strains) is indicated for treating disturbances of intestinal bacterial microbiota. B. clausii significantly improved symptoms in children with diarrhea [13, 14, 18,19,20,21,22,23,24,25,26,27]. In a study in children (6–12 years, n = 15) with IBS, B. clausii plus conventional therapy resulted in significantly more patients with overall symptom improvement and bowel movement normalization, significantly fewer patients with abdominal bloating, and significantly lower pain intensity and number of pain events versus conventional treatment [28]. This larger RCT, BaclauSII, aimed to fill a data gap for children with IBS.
There are several potential reasons why BaclauSII was not able to demonstrate an overall positive adjuvant effect with B. clausii versus placebo on IBS symptoms. BaclauSII was powered to detect a 20% difference between groups, thus, the very high placebo responses may have masked any additional benefit of B. clausii. Placebo effects occur in gastroenterology RCTs more than in other diseases [43]. Moreover, the placebo response in gastroenterology (visceral pain, nausea) has neuro- and psychobiological properties along the gut–brain axis [43], a key target for probiotics in IBS [1, 42]. In addition, BaclauSII was conducted under strictly controlled social conditions during the COVID-19 pandemic. Isolation at home is likely to have resulted in more diet control and greater family stability. A recent survey of adults with IBS reported a significant decrease in severe IBS symptoms during lockdown in the COVID-19 pandemic compared with pre-pandemic data, possibly due to reduced external stressors [44]. Diet directly modifies the composition of the intestinal microbiota [45]; thus, recommendations to follow a healthy diet according to age as part of the conventional treatment could have led to the improvement seen in the placebo group. Indeed, better adherence to the conventional therapy may also have occurred during social isolation in the COVID-19 pandemic, thereby also contributing to improved IBS symptoms in both groups; although adherence to conventional therapy was not assessed in this study.
Furthermore, the children were not exposed to school pressure, and had a lower risk of infections that could reduce IBS symptoms, including gastrointestinal diarrhea. Such favorable conditions may explain the excellent response to conventional therapy. Although these considerations are speculative, it remains to be defined whether the placebo results in BaclauSII would be reproducible in normal everyday life. Recent probiotic studies in functional gastrointestinal disorders are conducted in the context of improving global health outcomes, and demonstrating global improvements with probiotics is becoming more challenging.
Some significant differences were seen in BaclauSII. Median percentage of days with bloating was lower in patients with IBS-C in the B. clausii group versus placebo. Bloating is common in IBS [46]. However, two RCTs showed that other probiotics did not impact the rate of bloating versus placebo (relative risk 0.32; 95% CI 0.04–2.56) [40]. In addition, median number of abdominal pain episodes was lower with B. clausii compared with placebo at Week 4.
Various probiotic strains have been shown to have beneficial effects in children with IBS [47]. Escherichia coli strains significantly improved symptoms in children with chronic IBS [48]. Bacillus coagulans with prebiotics significantly improved response rate versus placebo in children with functional abdominal pain [49]. Trials with Bifidobacterium strains have demonstrated significant abdominal pain reduction [50], and significantly improved belching, abdominal fullness, bloating, and constipation [51] in children with IBS. Various Lactobacillus strains had positive benefits in children with IBS in several RCTs [52,53,54,55,56]. An RCT with a probiotic mixture of Lactobacillus and Bifidobacterium strains demonstrated superiority versus placebo for symptom relief and had significant effects on abdominal pain/discomfort, abdominal bloating/gassiness, and life disruption [57]. In a meta-analysis of RCTs of probiotics in children, abdominal pain score, abdominal pain treatment success, frequency of abdominal pain, and standard abdominal pain were significantly reduced compared with placebo, although abdominal pain relief was not significant between probiotics and placebo [40]. BaclauSII had similar observations on reductions in abdominal pain and bloating among IBS patients taking B. clausii; although these were secondary endpoints in this study, and the primary endpoint was not significant. Thus, evidence is growing on the positive impact of probiotics in children with IBS. Evidence-based global guidelines from the World Gastroenterology Organization recommend specific probiotic strains for certain gastrointestinal disorders, including IBS [58].
A key challenge in understanding probiotic effects is study heterogeneity, making it difficult to compare effects of different strains and mixtures. Probiotic beneficial effects in IBS may be strain-specific [59]. Such strain-specificity may reflect different mechanisms impacting the gut–brain axis [59, 60].
No unexpected safety findings were observed in BaclauSII. The type and incidence of TEAEs observed were similar between groups. B. clausii is well tolerated in studies in children with diarrhea [13, 19,20,21,22, 61].
Moreover, the BaclauSII study also has some limitations. Duration of treatment is important in assessing outcomes in IBS. Certain treatments, such as the antispasmodic otilonium bromide, have beneficial effects within 10–15 weeks of treatment [62] whereas low FODMAP diets can reduce IBS symptoms within 11 days to 3 weeks [63]. As the patients received their ongoing IBS treatment during the 2-week run-in period, beneficial effects of such treatments may have shown up during the 8-week study period with B. clausii or placebo with conventional treatment, thereby potentially masking the potential benefit of B. clausii treatment. Although treatment was close to 8 weeks and treatment compliance was > 80% in most patients, the PP population only included 146 patients, mainly due to treatment non-compliance. However, this low treatment compliance was due to some diary cards being completed incorrectly as the treatment accountability (based on actual number of vials consumed) was high. This sizeable non-compliance might have impacted detection of treatment differences. IBS is heterogenous and may result in different symptoms across a population and diet may contribute to this heterogeneity [64]. Indeed, in BaclauSII, heterogeneity was noted as evidenced by different drug classes used to manage IBS symptoms. Thus, BaclauSII may not have been sufficiently powered to account for these factors. Further targeted investigations are recommended.
Whatever the reason(s) why recent RCTs have not identified B. clausii positive benefits in children with gastrointestinal disorders, which is reflected by the paucity of top-level evidence from probiotic trials [59], more studies with larger patient numbers are required with various probiotic doses and longer treatments to better determine the benefits of B. clausii, particularly in detecting any efficacy differences that might be related to different societies and healthcare environments. The current BaclauSII trial did not report many significant improvements in IBS-related parameters, most likely due to the high placebo effect, as described above. An average placebo effect of 40–50% is typically expected in IBS studies [65, 66]. Indeed, the sample size calculation for BaclauSII was based on a treatment success of 45% in the placebo group [35] and an expected difference of 20% between the groups. However, much higher placebo effects of 70–80% have been reported [66, 67], a finding which is supported by BaclauSII. Therefore, the sample size should have been much larger in BaclauSII to show a favorable effect of B. clausii. In addition, due to IBS heterogeneity, data from real-world studies may also be valuable in identifying B. clausii benefits in IBS.
5 Conclusion
This study between the B. clausii and placebo treatment groups was not able to demonstrate the efficacy of B. clausii as an adjuvant to conventional treatment of patients with IBS. There were favorable observations at some time points in the B. clausii group, particularly for abdominal pain and bloating. Overall, any potential benefit of B. clausii in this trial may have been masked by the high placebo effect, the controlled environment under a COVID-19 pandemic lockdown, education received by patients/guardians during enrollment about IBS and the importance of diet, and the understanding of the mechanisms of pain and how to deal with symptoms related to IBS. Thus, further investigations in larger and more targeted controlled trials are necessary.
References
Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: current knowledge, challenges and opportunities. World J Gastroenterol. 2018;24(21):2211–35. https://doi.org/10.3748/wjg.v24.i21.2211.
Sun Y, Li L, Xie R, Wang B, Jiang K, Cao H. Stress triggers flare of inflammatory bowel disease in children and adults. Front Pediatr. 2019;7:432. https://doi.org/10.3389/fped.2019.00432.
Ballou S, McMahon C, Lee HN, Katon J, Shin A, Rangan V, et al. Effects of irritable bowel syndrome on daily activities vary among subtypes based on results from the IBS in America survey. Clin Gastroenterol Hepatol. 2019;17(12):2471–8. https://doi.org/10.1016/j.cgh.2019.08.016.
Singh R, Salem A, Nanavati J, Mullin GE. The role of diet in the treatment of irritable bowel syndrome: a systematic review. Gastroenterol Clin North Am. 2018;47(1):107–37. https://doi.org/10.1016/j.gtc.2017.10.003.
Chen L, Ilham SJ, Feng B. Pharmacological approach for managing pain in irritable bowel syndrome: a review article. Anesth Pain Med. 2017;7(2):e42747. https://doi.org/10.5812/aapm.42747.
Alammar N, Wang L, Saberi B, Nanavati J, Holtmann G, Shinohara RT, et al. The impact of peppermint oil on the irritable bowel syndrome: a meta-analysis of the pooled clinical data. BMC Complement Altern Med. 2019;19(1):21. https://doi.org/10.1186/s12906-018-2409-0.
Pimentel M. Evidence-based management of irritable bowel syndrome with diarrhea. Am J Manag Care. 2018;24(3 Suppl):S35-46.
Jadallah KA, Kullab SM, Sanders DS. Constipation-predominant irritable bowel syndrome: a review of current and emerging drug therapies. World J Gastroenterol. 2014;20(27):8898–909. https://doi.org/10.3748/wjg.v20.i27.8898.
Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol. 2019;114(1):21–39. https://doi.org/10.1038/ajg.2014.148.
Gu Y, Zhou G, Qin X, Huang S, Wang B, Cao H. The potential role of gut mycobiome in irritable bowel syndrome. Front Microbiol. 2019;10:1894. https://doi.org/10.3389/fmicb.2019.01894.
Ghelardi E, Celandroni F, Salvetti S, Gueye SA, Lupetti A, Senesi S. Survival and persistence of Bacillus clausii in the human gastrointestinal tract following oral administration as spore-based probiotic formulation. J Appl Microbiol. 2015;119(2):552–9. https://doi.org/10.1111/jam.12848.
Gabrielli M, Lauritano EC, Scarpellini E, Lupascu A, Ojetti V, Gasbarrini G, et al. Bacillus clausii as a treatment of small intestinal bacterial overgrowth. Am J Gastroenterol. 2009;104(5):1327–8. https://doi.org/10.1038/ajg.2009.91.
Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, et al. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335(7615):340. https://doi.org/10.1136/bmj.39272.581736.55.
Lahiri K, Jadhav K, Gahlowt P, Najmuddin F. Bacillus clausii as an adjuvant therapy in acute childhood diarrhoea. J Den Med Sci. 2015;14(5):74–6.
Pochapin M. The effect of probiotics on Clostridium difficile diarrhea. Am J Gastroenterol. 2000;95(1 Suppl):S11-13. https://doi.org/10.1016/s0002-9270(99)00809-6.
Nista EC, Candelli M, Cremonini F, Cazzato IA, Zocco MA, Franceschi F, et al. Bacillus clausii therapy to reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-blind, placebo controlled trial. Aliment Pharmacol Ther. 2004;20(10):1181–8. https://doi.org/10.1111/j.1365-2036.2004.02274.x.
Plomer M, Perez M, Greifenberg DM. Effect of Bacillus clausii capsules in reducing adverse effects associated with Helicobacter pylori eradication therapy: A randomized, double-blind, controlled trial. Infect Dis Ther. 2020;9(4):867–78. https://doi.org/10.1007/s40121-020-00333-2.
Plomer M, Perez M, Arnotfalvy B, Vargas D, Lam HY, Uboldi M-C. The probiotic Bacillus clausii in the prevention of antibiotic-associated diarrhoea in children: a pooled analysis of controlled clinical trials. J Pediatr Gastroenterol Nutr. 2019;68(1 Suppl):478. https://doi.org/10.13140/RG.2.2.32794.26562.
Maity C, Gupta AK. Therapeutic efficacy of probiotic Alkalihalobacillus clausii 088AE in antibiotic-associated diarrhea: a randomized controlled trial. Heliyon. 2021;7(9):e07993. https://doi.org/10.1016/j.heliyon.2021.e07993.
Hamid F, Moosa S, Quaium MA, Rahman A. Comparative study of Bacillus clausii and multistrain probiotics in the management of acute diarrhoea in children. Int J Res Med Sci. 2019;7(4):1156–60. https://doi.org/10.18203/2320-6012.ijrms20191317.
Sudha MR, Jayanthi N, Pandey DC, Verma AK. Bacillus clausii UBBC-07 reduces severity of diarrhoea in children under 5 years of age: a double blind placebo controlled study. Benef Microbes. 2019;10(2):149–54. https://doi.org/10.3920/bm2018.0094.
de Castro JA, Guno MJV, Perez MO. Bacillus clausii as adjunctive treatment for acute community-acquired diarrhea among Filipino children: a large-scale, multicenter, open-label study (CODDLE). Trop Dis Travel Med Vaccines. 2019;5:14. https://doi.org/10.1186/s40794-019-0089-5.
Acevedo NC, Fernandez FR, Moreira ED, Sano F, Bottino MG, Vázquez-Frias R. CadiLAc study: Bacillus clausii as an adjuvant therapy in acute community-acquired diarrhoea among Latin American children. J Pediatr Gastroenterol Nutr. 2019;68(1 Suppl):481.
Ianiro G, Rizzatti G, Plomer M, Lopetuso L, Scaldaferri F, Franceschi F, et al. Bacillus clausii for the treatment of acute diarrhea in children: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2018;10(8):1074. https://doi.org/10.3390/nu10081074.
McFarland L, Srinivasan R, Setty R, Ganapathy S, Bavdekar A, Mitra M, et al. Specific probiotics for the treatment of pediatric acute gastroenteritis in India: a systematic review and meta-analysis. JPGN Reports. 2021;2(3):e079.
Smiyan OI, Smiian-Horbunova KO, Bynda TP, Loboda AM, Popov SV, Vysotsky IY, et al. Optimization of the treatment of rotavirus infection in children by using Bacillus clausii. Wiad Lek. 2019;72(7):1320–3.
Smiian K, Smiyan O, Bynda T, Loboda A. Bacillus clausii in treatment of rotavirus infection in children. Medicina (Kaunas). 2020;56(1 Suppl):225.
Acosta-Rodríguez-Bueno CP, Vázquez-Frias R, Consuelo-Sánchez A, Urban M. Effectiveness of Bacillus clausii as an adjuvant treatment for pediatric irritable bowel syndrome: pretest-posttest design. J Pediatr Gastroenterol Nutri. 2020;71(1 Suppl):S48 (Abstract 72).
Food and Drug Administration. Guidance for industry: irritable bowel syndrome—clinical evaluation of products for treatment. May 2021. https://www.fda.gov/media/78622/download. Accessed 21 July 2022.
Saps M, van Tilburg MA, Lavigne JV, Miranda A, Benninga MA, Taminiau JA, et al. Recommendations for pharmacological clinical trials in children with irritable bowel syndrome: the Rome foundation pediatric subcommittee on clinical trials. Neurogastroenterol Motil. 2016;28(11):1619–31. https://doi.org/10.1111/nmo.12896.
Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, van Tilburg M. Functional disorders: children and adolescents. Gastroenterology. 2016. https://doi.org/10.1053/j.gastro.2016.02.015.
Senesi S, Celandroni F, Tavanti A, Ghelardi E. Molecular characterization and identification of Bacillus clausii Strains marketed for use in oral bacteriotherapy. Appl Environ Microbiol. 2001;67(2):834–9. https://doi.org/10.1128/AEM.67.2.834-839.2001.
Miller LE. Study design considerations for irritable bowel syndrome clinical trials. Ann Gastroenterol. 2014;27(4):338–45.
Mohammad S, Di Lorenzo C, Youssef NN, Miranda A, Nurko S, Hyman P, Saps M. Assessment of abdominal pain through global outcomes and recent FDA recommendations in children: are we ready for change? J Pediatr Gastroenterol Nutr. 2014;58(1):46–50. https://doi.org/10.1097/MPG.0b013e3182a20764.
Saps M, Youssef N, Miranda A, Nurko S, Hyman P, Cocjin J, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology. 2009;137(4):1261–9. https://doi.org/10.1053/j.gastro.2009.06.060.
Ida M, Nishida A, Akiho H, Nakashima Y, Matsueda K, Fukudo S. Evaluation of the irritable bowel syndrome severity index in Japanese male patients with irritable bowel syndrome with diarrhea. Biopsychosoc Med. 2017;11:7. https://doi.org/10.1186/s13030-017-0092-x.
EMA. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. EMA/CHMP/60337/2013. June 2013. https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-evaluation-medicinal-products-treatment-irritable-bowel-syndrome-revision-1_en.pdf. Accessed 2 Aug 2022.
Müller-Lissner S, Koch G, Talley NJ, Drossman D, Rueegg P, Dunger-Baldauf C, et al. Subject’s Global Assessment of Relief: an appropriate method to assess the impact of treatment on irritable bowel syndrome-related symptoms in clinical trials. J Clin Epidemiol. 2003;56(4):310–6. https://doi.org/10.1016/S0895-4356(03)00027-1.
Kerckhove N, Scanzi J, Pereira B, Ardid D, Dapoigny M. Assessment of the effectiveness and safety of ethosuximide in the treatment of abdominal pain related to irritable bowel syndrome - IBSET: protocol of a randomised, parallel, controlled, double-blind and multicentre trial. BMJ Open. 2017;7(7):e015380. https://doi.org/10.1136/bmjopen-2016-015380.
Xu HL, Zou LL, Chen MB, Wang H, Shen WM, Zheng QH, et al. Efficacy of probiotic adjuvant therapy for irritable bowel syndrome in children: a systematic review and meta-analysis. PLoS ONE. 2021;16(8):e0255160. https://doi.org/10.1371/journal.pone.0255160.
McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14(17):2650. https://doi.org/10.3748/wjg.14.2650.
Singh P, Lembo A. Emerging role of the gut microbiome in irritable bowel syndrome. Gastroenterol Clin N Am. 2021;50(3):523–45. https://doi.org/10.1016/j.gtc.2021.03.003.
Elsenbruch S, Enck P. Placebo effects and their determinants in gastrointestinal disorders. Nat Rev Gastroenterol Hepatol. 2015;12(8):472–85. https://doi.org/10.1038/nrgastro.2015.117.
Piskorz M, Stefanolo JP, Ibañez A, Hesse E, Bravo Velez G, Tevez A, et al. Gut-brain axis and irritable bowel syndrome during Sars Cov-2 pandemic. A survey based study. Gastroenterology. 2021;160(6 Supplement):S615 (abstract Su093).
Leeming ER, Johnson AJ, Spector TD, Le Roy CI. Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients. 2019;11(12):2862. https://doi.org/10.3390/nu11122862.
Pop LL, Mureşan IA, Dumitraşcu DL. How much bloating in the irritable bowel syndrome? Rom J Intern Med. 2018;56(4):221–6. https://doi.org/10.2478/rjim-2018-0017.
Brusaferro A, Farinelli E, Zenzeri L, Cozzali R, Esposito S. The management of paediatric functional abdominal pain disorders: latest evidence. Paediatr Drugs. 2018;20(3):235–47. https://doi.org/10.1007/s40272-018-0287-z.
Martens U, Enck P, Zieseniss E. Probiotic treatment of irritable bowel syndrome in children. Ger Med Sci. 2010;8:Doc07. https://doi.org/10.3205/000096.
Saneian H, Pourmoghaddas Z, Roohafza H, Gholamrezaei A. Synbiotic containing Bacillus coagulans and fructo-oligosaccharides for functional abdominal pain in children. Gastroenterol Hepatol Bed Bench. 2015;8(1):56–65.
Giannetti E, Maglione M, Alessandrella A, Strisciuglio C, De Giovanni D, Campanozzi A, et al. A mixture of 3 Bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover Trial. J Clin Gastroenterol. 2017;51(1):e5-10. https://doi.org/10.1097/mcg.0000000000000528.
Baştürk A, Artan R, Yılmaz A. Efficacy of synbiotic, probiotic, and prebiotic treatments for irritable bowel syndrome in children: a randomized controlled trial. Turk J Gastroenterol. 2016;27(5):439–43. https://doi.org/10.5152/tjg.2016.16301.
Gawrońska A, Dziechciarz P, Horvath A, Szajewska H. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177–84. https://doi.org/10.1111/j.1365-2036.2006.03175.x.
Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33(12):1302–10. https://doi.org/10.1111/j.1365-2036.2011.04665.x.
Romano C, Ferrau’ V, Cavataio F, Iacono G, Spina M, Lionetti E, et al. Lactobacillus reuteri in children with functional abdominal pain (FAP). J Paediatr Child Health. 2014;50(10):E68-71. https://doi.org/10.1111/j.1440-1754.2010.01797.x.
Jadrešin O, Hojsak I, Mišak Z, Kekez AJ, Trbojević T, Ivković L, et al. Lactobacillus reuteri DSM 17938 in the treatment of functional abdominal pain in children: RCT study. J Pediatr Gastroenterol Nutr. 2017;64(6):925–9. https://doi.org/10.1097/mpg.0000000000001478.
Weizman Z, Abu-Abed J, Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2016;174:160–4. https://doi.org/10.1016/j.jpeds.2016.04.003.
Guandalini S, Magazzù G, Chiaro A, La Balestra V, Di Nardo G, Gopalan S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51(1):24–30. https://doi.org/10.1097/mpg.0b013e3181ca4d95.
World Gastroenterology Organisation Global Guidelines. Probiotics and prebiotics. February 2017. https://www.worldgastroenterology.org/UserFiles/file/guidelines/probiotics-and-prebiotics-english-2017.pdf. Accessed 7 Mar 2022.
Korpela R, Niittynen L. Probiotics and irritable bowel syndrome. Microb Ecol Health Dis. 2012. https://doi.org/10.3402/mehd.v23i0.18573.
Lee BJ, Bak YT. Irritable bowel syndrome, gut microbiota and probiotics. J Neurogastroenterol Motil. 2011;17(3):252–66. https://doi.org/10.5056/jnm.2011.17.3.252.
Lahiri KR, Singh R, Apte M, Patil M, Taksande A, Varona R, et al. Efficacy and safety of Bacillus clausii (O/C, N/R, SIN, T) probiotic combined with oral rehydration therapy (ORT) and zinc in acute diarrhea in children: a randomized, double-blind, placebo-controlled study in India. Trop Dis Travel Med Vaccines. 2022;8(1):9. https://doi.org/10.1186/s40794-022-00166-6.
Clavé P, Tack J. Efficacy of otilonium bromide in irritable bowel syndrome: a pooled analysis. Therap Adv Gastroenterol. 2017;10(3):311–22. https://doi.org/10.1177/1756283X16681708.
Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9(9):940. https://doi.org/10.3390/nu9090940.
Algera J, Colomier E, Simrén M. The dietary management of patients with irritable bowel syndrome: a narrative review of the existing and emerging evidence. Nutrients. 2019;11(9):2162. https://doi.org/10.3390/nu11092162.
Lu CL, Chang FY. Placebo effect in patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26(3 Suppl):116–8. https://doi.org/10.1111/j.1440-1746.2011.06651.x.
Enck P, Klosterhalfen S. Placebo responses and placebo effects in functional gastrointestinal disorders. Front Psychiatry. 2020;11:797. https://doi.org/10.3389/fpsyt.2020.00797.
Lembo A, Kelley JM, Nee J, Ballou S, Iturrino J, Cheng V, et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain. 2021;162(9):2428–35. https://doi.org/10.1097/j.pain.0000000000002234.
Acknowledgments
The authors thank all of the patients and their parents/guardians for their contribution to this study. The authors would also like to thank the other Investigators in Mexico who contributed to this study: Francisco Benitez (ICARO Investigaciones en Medicina S.A. de C.V.), Josefina Cazares (Phylasis Clinicas Research S de RL de CV), Maria Galaviz (USIP, Unidad de Investigacion y Salud de Puebla SC), Roxanna Garcia (Centro de Investigacion Clinica Acelerada SC), Gabriela Hernández (Hospital San Lucas), Karen Ignorosa (Arké Estudios Clinicos S.A. de C.V.), Irene Jackson (Arké Estudios Clinicos S.A. de C.V.), Maria Ortal (UISP, Unidad de Investigacion y Salud de Puebla SC), Francisco Salazar (FACIC S. DE R.L. DE C.V.), Martha Sanchez (Centro de Estudios Clinicos de Querétaro S.C.), and Claudia Sifuentes (Centro de Desarrollo Biomédico). Medical writing and editorial support for this manuscript were provided by Jackie Phillipson and Malgorzata Urbacz, of Ashfield MedComms, an Inizio company, and was funded by Sanofi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access was funded by Sanofi. This study was funded by Sanofi.
Conflicts of interest/Competing interests
Rodrigo Vázquez-Frias has received research grants from Sanofi; received consulting fees from BioGaia, Carnot, Nestle, and Nestle Nutrition Institute; and has received speaker honorarium from Abbot Pharmaceuticals, BioGaia, Carnot, Ferrer, Nestle, Nestle Nutrition Institute, and Schwabe Pharma. Alejandra Consuelo-Sánchez has participated in advisory boards for Sanofi and academic events organized by Sanofi; received some payment for conducting procedures in this study (as per his previous contract with his hospital); and has assisted in National and International Congresses (pre-2019). Carlos Patricio Acosta-Rodríguez-Bueno declares no conflict of interest. Andrés Blanco-Montero received a payment as part of the research team that conducted this study. Daniel Casas Robles received payment for participating in this study research. Vanessa Cohen, Daniel Márquez, and Marcos Perez III are current employees of Sanofi and may hold shares and/or stock options in the company.
Ethics
The protocol was approved by Independent Ethics Committees and Research Committees. The study was conducted in accordance with all applicable laws, rules, and regulations and with the Declaration of Helsinki and the International Council for Harmonisation guidelines for Good Clinical Practice.
Consent to participate
Parents/legal guardians provided written, informed consent on behalf of their child at the time of enrollment. An informed assent form was signed by the patient, if applicable (age of assent was determined by the Independent Ethics Committees and Research Committees).
Consent to publication
Not applicable.
Data availability
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.vivli.org/.
Code availability
Not applicable.
Author contributions
Rodrigo Vázquez-Frias, Alejandra Consuelo-Sánchez, Carlos Patricio Acosta-Rodríguez-Bueno, Vanessa Cohen, Daniel Márquez, and Marcos Perez III contributed to study conception and methodology. Validation was performed by Rodrigo Vázquez-Frias. Investigations were conducted by Rodrigo Vázquez-Frias, Alejandra Consuelo-Sánchez, Carlos Patricio Acosta-Rodríguez-Bueno, Andrés Blanco-Montero, and Daniel Casas Robles. Carlos Patricio Acosta-Rodríguez-Bueno, Vanessa Cohen, Daniel Márquez, and Marcos Perez III contributed resources. Data curation was provided by Alejandra Consuelo-Sánchez, and Daniel Casas Robles. Supervision was provided by Rodrigo Vázquez-Frias, Vanessa Cohen, Daniel Márquez, and Marcos Perez III. Vanessa Cohen, Daniel Márquez, and Marcos Perez III contributed to project administration and funding acquisition. All authors contributed to writing (review and edit) of the paper, and read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vázquez-Frias, R., Consuelo-Sánchez, A., Acosta-Rodríguez-Bueno, C.P. et al. Efficacy and Safety of the Adjuvant Use of Probiotic Bacillus clausii Strains in Pediatric Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Study. Pediatr Drugs 25, 115–126 (2023). https://doi.org/10.1007/s40272-022-00536-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-022-00536-9