Abstract
Background
Presinusoidal portal hypertension is a clinically important cause of gastric and gastroesophageal varices. Whereas β-blockers have an established prophylactic role against bleeding from esophageal and gastric varices in portal hypertension due to cirrhosis, the effect on presinusoidal portal hypertension is unknown.
Aims
To evaluate the hemodynamic effect of β-blockers in non-cirrhotic patients with presinusoidal portal hypertension.
Methods
We measured the blood pressure gradient from spleen pulp to free hepatic vein in 12 patients with presinusoidal portal hypertension by combined hepatic vein catheterization and spleen pulp puncture while on and off β-blocker treatment (random sequence).
Results
The β-blockers reduced the gradient from a mean off-treatment value of 32 mm Hg to a on-treatment value of 26 mm Hg (P < 0.05) with a reduction of at least 20% in five patients (42%).
Conclusions
β-blocker treatment caused a clinically significant reduction in the pressure gradient from spleen pulp to the free hepatic vein. This finding supports the recommendation for prophylactic β-blockage in patients with presinusoidal portal hypertension.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
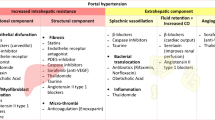
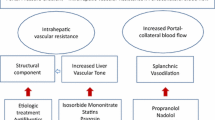
Presinusoidal portal hypertension is a rare but clinically important cause of bleeding gastric and gastroesophageal varices that can be difficult to manage. In contrast to the well-established role of nonselective β-blockers as primary and secondary prophylaxis against variceal bleeding in patients with cirrhosis and intrahepatic portal hypertension, there is no study addressing the hemodynamic effect of β-blockage on presinusoidal portal hypertension, viz., the pressure gradient between the spleen pulp and the free hepatic vein. Clinical recommendations and guidelines, such as the Baveno VI Consensus Workshop, pragmatically recommend patients with prehepatic portal hypertension be treated with β-blockers as secondary prophylaxis while acknowledging the lack of evidence for the recommendation [1, 2].
The hemodynamic basis for the recommendations for patients with cirrhosis is hepatic vein catheterizations with measurements of the effect of β-blockers on the hepatic venous pressure gradient (HVPG) [3,4,5,6]. In presinusoidal portal hypertension, the crucial blood pressure gradient is upstream to the liver which can be measured by combined hepatic vein catheterization and direct percutaneous, thin-needle puncture of the spleen [7]. We use this technique as a clinical procedure and have found it to be safe [8] in agreement with a recent meta-analysis that found the complication rate from thin-needle spleen biopsies to be as low as that of liver and kidney biopsies [9].
Using traditional hepatic vein catheterization in combination with direct spleen pulp puncture, we prospectively evaluated the effect of treatment with β-blockers on the spleen to free hepatic vein pressure gradient in patients with presinusoidal portal hypertension by measurements off and on β-blocker treatment.
Materials and Methods
Patients
This is a prospective on- and off-treatment case series of 12 non-cirrhotic patients (three female and nine male; median age of 49 years, range 22–79) with presinusoidal portal hypertension. All were normoweight based on body mass index (20–27 kg/m2 body surface) except ID 8 (44 kg/m2 body surface). Clinical characteristics are given in Table 1, and all diagnoses were based on standard clinical workup. All patients had clinically stable disease. Three patients (IDs 2, 9, and 10) had presinusoidal portal hypertension due to intrahepatic obstruction, while the remaining nine had prehepatic obstruction. Individual causes and etiology are given in Table 1. Type and degree of varices are also shown; four patients had experienced bleeding episodes from the varices, eight patients received anticoagulant therapy, and five patients were treated endoscopically (banding). Based on clinical risk assessment and in accordance with the prevailing guidelines [3], we offered the patients a treatment with β-blockers in an attempt to reduce the risk of index or repeat bleeding episodes. Dosage was 80 mg propranolol/day.
The pressure measurements were performed after an overnight fast with catheterization of the hepatic vein and direct spleen pulp puncture. The procedures were conducted according to our clinical guidelines as described below. Initiation of the treatment did not await the hemodynamic measurement, and if started before the first measurement, it was paused for 5 days before the procedure. Thus, in ten patients, the first investigation was off β-blocker treatment (four of the patients had already started treatment with β-blocker and therefore paused treatment for the first measurement), and median time between the two measurements was 4 months (range 1.5–18 months). Other medications (such as diuretics) were dose-adjusted as necessary between the investigations, but treatments were neither initiated nor stopped.
None of the patients underwent splenectomy before or during the treatment.
Ethics
The procedures were conducted clinically to evaluate the hemodynamic effect of β-blocker treatment in the same way as our clinical management of patients with cirrhosis and portal hypertension. In patients who paused β-blocker treatment for the off-treatment measurement, we considered the infinitesimal risk of withholding the treatment for 5 days to be outweighed by the benefit of avoidance of non-efficacious treatment and its potential side effects. We did not experience significant complications to the spleen puncture procedure conducted as described below. The data we present were retrieved from the clinical records of the procedures and from the electronic patient records. As with all the other clinical procedures, the patients were carefully informed about risks and benefits beforehand.
Hepatic Vein Catheterization
With the patient in supine position, a 6.7-F catheter of the Cournand type (Cook, Denmark) was inserted into a right hepatic vein through an introducer placed percutaneously in the right femoral vein. The tip of the catheter was advanced into the wedged position and the pressure was measured. The tip was then retracted to the free position for pressure measurement. The position of the tip was verified by fluoroscopy and injection of X-ray contrast (5–20 mL Omnipaque 240 mg I/mL; Nycomed DAK, Denmark).
Spleen Pulp Puncture
The patient tilted slightly to the right with the left arm over the head. The spleen was visualized by ultrasound, and local infiltration anesthesia of the skin and peritoneum was applied (Lidocain, 10 mg/mL, 5 mL; Danish Hospitals’ Pharmacy, Copenhagen). The puncture site was just below the left curvature of the ribs. The patient held his/her breath for up to 20 s, while a 20-G 12-cm-long needle (diameter 0.9 mm, Mediplast, Japan) was introduced into the spleen in a cephalad direction at an angle of 30° to the transverse plane in order to minimize the risk of lacerating the spleen if the patient accidently took a deep breath during the procedure [6, 7]. The needle was inserted under ultrasonic guidance and advanced into the spleen until the tip was located in the central part of the parenchyma where the pressure was recorded.
Statistical Analysis
Intra-individual difference between on- and off-medication pressures was tested by a paired t test of the mean difference. A P value < 0.05 was considered an indication of a statistically significant effect of treatment. On the individual level, we considered a pressure gradient change of > 5% to be detectable. Due to the homogenous nature of this clinical data set, a sensitivity analysis was performed. This considered the potential influence of including patients with portal versus splenic vein thrombosis and the potential effect of treatment on HVPG; none of these factors affected the statistical results, and the patients are therefore presented as one group.
Results
Individual changes in pressure gradient are shown in Table 1. For the group as a total, the β-blocker treatment was associated with a mean decrease in spleen to free hepatic vein pressure gradient of 14% with a mean gradient off treatment of 32.0 mm Hg (range 24.4–55.0 mm Hg) versus 25.5 mm Hg (range 15.5–40.8 mm Hg) on treatment (P < 0.05) (Fig. 1). The gradient decreased in nine patients and was unchanged in three. Five patients (42%) obtained a gradient reduction of at least 20%.
The mean off-treatment HVPG was 4.0 mm Hg (range 1.0–9.0 mm Hg) and was unaffected by β-blockade (P = 0.97).
The mean off-treatment pressure in the spleen pulp was 34.5 mm Hg (range 25.6–50.0 mm Hg) which decreased on treatment to 28.6 mm Hg (range 16.3–34.7 mm Hg) (P < 0.05). In six patients (50%), the pressure in spleen pulp decreased by at least 20% on treatment when compared to off treatment (P < 0.05).
There were no statistical relationships between treatment effect and propranolol dose/BMI, heart rate, or arterial blood pressure (P > 0.30 for all, data not shown).
Discussion
The main result of this first systematic study of the hemodynamic effects of β-blockers on presinusoidal portal hypertension is that the treatment caused a significant reduction in the pressure gradient from spleen to the free hepatic vein position.
Current treatment options for patients with newly identified thrombosis in the portal or splenic vein are limited to anticoagulant therapy, trans-vascular thrombectomy, or, in highly selected cases, surgery [1, 10,11,12,13,14]. For patients with intrahepatic presinusoidal portal hypertension, we have no treatment. We believe that our findings provide pathophysiologic rationale to the pragmatic recommendation of treating patients with presinusoidal portal hypertension with β-blocker as primary and secondary prophylaxis against variceal bleeding [2,3,4,5,6]. The findings may also explain the beneficial effect of propranolol on recurrent bleeding episodes in patients with non-cirrhotic portal fibrosis due to schistosomiasis [15].
To what extent the hemodynamic effect of the β-blockage translates into the desired clinical effects, i.e., a decrease in the number of bleeding episodes, requires larger long-term observational studies, and for a definitive recommendation, randomized controlled trials are needed. Such trials will be difficult to conduct because of the rarity of the condition, and because not all patients with presinusoidal portal hypertension develop bleeding varices, a high number of patients will be needed. However, we do believe that our results motivate such a multicenter trial.
Atkinson and Sherlock found the spleen pulp pressure in normal man to be 10 mm Hg with an upper normal value of 17 mm Hg [7]. Off treatment, all our patients were above this upper threshold, confirming prehepatic portal hypertension. The risk of bleeding is determined by the intra-variceal pressure [10], which in case of presinusoidal portal pressure is determined by the reported pressure gradient. A pressure gradient reduction of at least 20% is desirable, if analogous to the situation in cirrhosis, and this was observed in 5/12 patients, viz., 42%.
Propranolol is shown to decrease the portal pressure by lowering the splanchnic inflow in portal vein-constricted rats, but a concomitant increase in portocollateral resistance counteracted the decrease in portal pressure [16]. This was not the case in rats with CCl4-induced cirrhosis and mild portosystemic shunting [17]. The extent of portosystemic shunting in patients with presinusoidal portal hypertension might thus diminish the response to propranolol. However, we did not find any clinical evidence of a more pronounced portosystemic shunting in the nonresponders in our patients. Furthermore, we found the same response rate (42%) as we did for the postsinusoidal portal hypertension in patients with cirrhosis [4].
In conclusion, we observed a clinically meaningful reduction in the blood pressure gradient from spleen pulp to the free hepatic vein by β-blocker treatment in patients with presinusoidal portal hypertension. Our data thus support the guideline recommendations of offering this treatment to such patients, but documentation of its clinical outcome benefit awaits studies with clinical end points.
References
Khanna R, Sarin SK. Non-cirrhotic portal hypertension—diagnosis and management. J Hepatol. 2014;60:421–441.
Kumar A, Sharma A, Arora A. Review article: portal vein obstruction—epidemiology, pathogenesis, natural history, prognosis and treatment. Aliement Pharmacol Ther. 2015;41:276–292.
de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762–768.
Heebøll S, Villadsen GE, Aagaard NK, Grønbøk H, Vilstrup H, Keiding S. Propranolol treatment of portal hypertension in cirrhosis patients is better the higher the untreated pressure: a single-centre prospective experience. Scand J Gastroenterol. 2013;48:969–973.
Bosch J, Abraldes JG, Berzigotti A, García-Pagan JC. The clinical use of HVPG measurements in chronic liver disease. Nat Rev Gastroenterol Hepatol. 2009;6:573–582.
Merkel C, Bolognesi M, Sacerdoti D, et al. The hemodynamic response to medical treatment of portal hypertension as a predictor of clinical effectiveness in the primary prophylaxis of variceal bleeding in cirrhosis. Hepatology. 2000;32:930–934.
Atkinson M, Sherlock S. Intrasplenic pressure as index of portal venous pressure. Lancet. 1953;263:1325–1327.
Keiding S, Solvig H, Grønbæk H, Vilstrup H. Combined liver vein spleen pulp pressure measurements in patients with portal or splenic vein thrombosis. Scand J Gastroenterol. 2004;39:594–599.
McInness MDF, Kielar AZ, Macdonald DB. Percutaneous image-guided biopsy of the spleen: systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology. 2011;260:699–708.
Polio J. Hemodynamic factors involved in the development and rupture of esophageal varices: a pathophysiological approach to treatment. Sem Liver Dis. 1986;6:318–331.
Riva N, Donadini MP, Dentali F, Squizzato A, Ageno W. Clinical approach to splanchnic vein thrombosis: risk factors and treatment. Thrombosis Res. 2012;130:S1–S3.
Glynn MJ. Isolated splenic vein thrombosis. Arch Surg. 1986;121:723–725.
Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell À. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919–928.
Henry Z, Uppal D, Saad W, Caldwell S. Gastric and ectopic varices. Clin Liv Dis. 2014;18:371–388.
Kiire CF. Controlled trial of propranolol to prevent recurrent variceal bleeding in patients with non-cirrhotic portal fibrosis. BMJ. 1989;298:1363–1365.
Kroeger RJ, Groszmann RJ. Increased portal venous resistance hinders portal pressure reduction during the administration of beta-adrenergic blocking agents in portal hypertensive model. Hepatology. 1985;5:97–101.
Pizcueta MP, De Lacy AM, Kravetz D, Bosch J, Rodés J. Propranolol decreases portal pressure without changing portocollateral resistance in cirrhotic rats. Hepatology. 1989;10:953–957.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of Interest.
Ethical Statement
For this type of study, formal consent is not required.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sørensen, M., Larsen, L.P., Villadsen, G.E. et al. β-Blockers Improve Presinusoidal Portal Hypertension. Dig Dis Sci 63, 3153–3157 (2018). https://doi.org/10.1007/s10620-018-5186-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-5186-1