Abstract
Background
Approximately 233,898 individuals in the Veterans Affairs healthcare network are hepatitis C virus (HCV)-infected, making the Veterans Affairs the single largest provider of HCV care in the USA. Direct-acting antiviral treatment regimens for HCV offer high cure rates. However, these medications pose an enormous financial burden, and whether HCV cure is associated with decreased healthcare costs is poorly defined.
Aims
To measure downstream healthcare costs in a national population of HCV-infected patients up to 9 years post-HCV antiviral treatment, to compare downstream healthcare costs between cured and uncured patients, and to assess impact of cirrhosis status on cost differences.
Methods
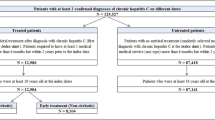
This is a retrospective cohort study (2004–2014) of hepatitis C-infected patients who initiated antiviral treatment within the United States Veterans Affairs healthcare system October 2004–September 2013. We measured inpatient, outpatient, and pharmacy costs after HCV treatment.
Results
For the entire cohort, cure was associated with mean cumulative cost savings in post-treatment years three–six, but no cost savings by post-treatment year nine. By post-treatment year nine, cure in cirrhosis patients was associated with a mean cumulative cost savings of $9474 (− 32,666 to 51,614) per patient, while cure in non-cirrhotic patients was associated with a mean cumulative cost excess of $2526 (− 12,211 to 7159) per patient.
Conclusions
Among patients with cirrhosis at baseline, cure is associated with absolute cost savings up to 9 years post-treatment compared to those without cure. Among patients without cirrhosis, early post-treatment cost savings are counterbalanced by higher costs in later years.



Similar content being viewed by others
References
Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003–2010. Ann Intern Med. 2014;160:293–300.
Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1992 through 2002. Ann Intern Med. 2006;144:705–714.
Maier M, Ross DB, Chartier M, Belperio PS, Backus LI. Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. Am J Public Health. 2016;106(2):353–358.
Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–1493.
Afdhal N, Zuezem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–1898.
The University of Washington, The University of Alabama at Birmingham, and the International Antiviral Society-USA. Hepatitis C online. Section editors: Spach DH, Kim HN. Available from: http://www.hepatitisc.uw.edu/page/treatment/drugs/ledipasvir-sofosbuvir. Accessed September 22, 2016.
Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928–936.
Linas BP, Barter DM, Morgan JR, et al. The cost-effectiveness of sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med. 2015;162:619–629.
Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406.
Hagan LM, Sulkowski MS, Schinazi RF. Cost analysis of sofosbuvir/ribavirin versus sofosbuvir/simeprevir for genotype 1 HCV in interferon ineligible/intolerant individuals. Hepatology. 2014;60(1):37–45.
Gordon SC, Hamzeh FM, Pockros PJ, et al. Hepatitis C virus therapy is associated with lower health care costs not only in noncirrhotic patients but also in patients with end-stage liver disease. Aliment Pharmacol Ther. 2013;38(7):784–793.
Manos MM, Darbinian J, Rubin J, et al. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm. 2013;19(6):438–447.
Kramer JR, Davila JA, Miller ED, Richardson R, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282.
Wang X, Beste LA, Maier MM, Zhou XH. Double robust estimator of average causal treatment effect for censored medical cost data. Stat Med. 2016;35(18):3101–3116.
Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–141.
Berenguer J, Alvarez-Pellicer J, Martín PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology. 2009;50(2):407–413.
Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308(4):370–378.
Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-term treatment outcomes of patients infected with hepatitis C virus: a systematic review and meta-analysis of the survival benefit of achieving a sustained virological response. Clin Infect Dis. 2015;61(5):730–740.
Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran K, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9(6):509–516.
Funding
No financial arrangements were related to the research. No outside assistance was received for manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This operational evaluation project was sponsored by the VA Office of HIV, Hepatitis, and Public Health Pathogens Program. The activities were undertaken in support of VA operational programs and did not constitute research, in whole or in part, in compliance with VA Handbook 1058.05. Therefore, institutional review board approval was not required.
Human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Informed consent
As our evaluation project was retrospective, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maier, M.M., Zhou, XH., Chapko, M. et al. Hepatitis C Cure Is Associated with Decreased Healthcare Costs in Cirrhotics in Retrospective Veterans Affairs Cohort. Dig Dis Sci 63, 1454–1462 (2018). https://doi.org/10.1007/s10620-018-4956-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-4956-0