Abstract
Aim
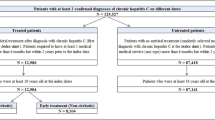
The recently developed direct-acting antivirals (DAAs) for hepatitis C virus (HCV) infections are costly. Cost-effectiveness analyses of DAAs require accurate healthcare expenditure estimates for the various HCV disease states, but few studies have produced such estimates using national-level data. This study utilized nationally representative data to estimate the healthcare expenditure for each HCV disease state.
Methods
We identified all patients infected with HCV between April 2010 and March 2018 from a nationwide administrative claims database in Japan. Monthly patient-level healthcare expenditures were calculated for the following disease states: chronic hepatitis C (CHC), compensated cirrhosis (CC), decompensated cirrhosis (DC), and hepatocellular carcinoma (HCC). The expenditures for the CHC and CC states were also compared before DAA treatment and after sustained virologic response (SVR) was achieved. A longitudinal two-part model was employed to estimate the healthcare expenditures for each state.
Results
During the study period, 1,564,043 patients with 146,488,137 patient-months of data met the inclusion criteria. The year of valuation was 2017. The mean monthly healthcare expenditures per patient (95% confidence intervals) for the pre-DAA CHC, CC, DC, and HCC states were US$267 (US$267–268), US$428 (US$427–429), US$666 (US$663–669), and US$969 (US$966–972), respectively. The mean monthly healthcare expenditures per patient for the post-SVR (≥ 2 years) CHC and CC states were US$176 (US$176–177) and US$238 (US$236–240), respectively. Healthcare expenditure increased with increasing age in all disease states (P < 0.05).
Conclusions
These healthcare expenditure estimates from a nationally representative sample have potential applications in cost-effectiveness analyses of DAAs.
Similar content being viewed by others
Data Availability Statement
The data that support the findings reported here were obtained from the Japanese Ministry of Health, Labour and Welfare under license for the current study, and are not publicly available due to restrictions stipulated by the ministry. However, the data are available from the Ministry of Health, Labour and Welfare upon reasonable request.
References
Ministry of Health, Labour and Welfare. Estimates of national medical care expenditure 2015. http://www.mhlw.go.jp/toukei/saikin/hw/k-iryohi/15/dl/kekka.pdf. Accessed 19 Sept 2019.
Ministry of Health, Labour and Welfare. Trends in medical care expenditure FY2016. http://www.mhlw.go.jp/topics/medias/year/16/dl/iryouhi_data.pdf. Accessed 19 Sept 2019.
Ministry of Health, Labour and Welfare. NDB Open Data Japan 2014. http://www.mhlw.go.jp/seisakunitsuite/bunya/0000139390.html. Accessed 19 Sept 2019.
Ministry of Health, Labour and Welfare. NDB Open Data Japan 2015. http://www.mhlw.go.jp/seisakunitsuite/bunya/0000177221.html. Accessed 19 Sept 2019.
Kumada H, Suzuki Y, Ikeda K, et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59(6):2083–91.
Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21(11):762–8.
Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15(6):645–53.
Chayama K, Notsumata K, Kurosaki M, et al. Randomized trial of interferon- and ribavirin-free ombitasvir/paritaprevir/ritonavir in treatment-experienced hepatitis C virus-infected patients. Hepatology. 2015;61(5):1523–32.
Kumada H, Chayama K, Rodrigues L Jr, et al. Randomized phase 3 trial of ombitasvir/paritaprevir/ritonavir for hepatitis C virus genotype 1b-infected Japanese patients with or without cirrhosis. Hepatology. 2015;62(4):1037–46.
Chahal HS, Marseille EA, Tice JA, et al. Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern Med. 2016;176(1):65–73.
Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397–406.
Chidi AP, Rogal S, Bryce CL, et al. Cost-effectiveness of new antiviral regimens for treatment-naïve U.S. veterans with hepatitis C. Hepatology. 2016;63(2):428–36.
Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–19.
Younossi ZM, Park H, Saab S, Ahmed A, Dieterich D, Gordon SC. Cost-effectiveness of all-oral ledipasvir/sofosbuvir regimens in patients with chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2015;41(6):544–63.
Virabhak S, Yasui K, Yamazaki K, et al. Cost-effectiveness of direct-acting antiviral regimen ombitasvir/paritaprevir/ritonavir in treatment-naïve and treatment-experienced patients infected with chronic hepatitis C virus genotype 1b in Japan. J Med Econ. 2016;19(12):1144–56.
McEwan P, Ward T, Webster S, et al. Estimating the cost-effectiveness of daclatasvir plus asunaprevir in difficult to treat Japanese patients chronically infected with hepatitis C genotype 1b. Hepatol Res. 2016;46(5):423–33.
Igarashi A, Tang W, Guerra I, Marie L, Cure S, Lopresti M. Cost-utility analysis of ledipasvir/sofosbuvir for the treatment of genotype 1 chronic hepatitis C in Japan. Curr Med Res Opin. 2017;33(1):11–21.
Wisloff T, White R, Dalgard O, et al. Economic evaluation of direct-acting antivirals for hepatitis C in Norway. Pharmacoeconomics. 2018;36(5):591–601.
McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17(7):531–46.
Ikeda S, Kobayashi M. Research on health-economics of various initiatives related to viral liver diseases, Ministry of Health, Labour and Welfare, Japan (PI: Hirao T) 2014;119–121. Accessed 10 Feb 2018. (in Japanese).
Ikeda S. Research on health-economics of various initiatives related to viral liver diseases, Ministry of Health, Labour and Welfare, Japan (PI: Hirao T) 2015;24–28. Accessed 10 Feb 2018. (in Japanese).
Ikeda S. Research on health-economics of various initiatives related to viral liver diseases, Ministry of Health, Labour and Welfare, Japan (PI: Hirao T) 2016;22–31. Accessed 10 Feb 2018. (in Japanese).
Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001;20(4):461–94.
Fukuda H, Morikane K, Kuroki M, et al. Impact of surgical site infections after open and laparoscopic colon and rectal surgeries on postoperative resource consumption. Infection. 2012;40(6):649–59.
Smith VA, Maciejewski ML, Olsen MK. Modeling semicontinuous longitudinal expenditures: a practical guide. Health Serv Res. 2018;53(Suppl 1):3125–47.
Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016;65(4):719–26.
Committee for Guideline Development for Hepatitis Treatment, The Japan Society of Hepatology. Treatment guidelines for hepatitis C (sixth edition). https://www.jsh.or.jp/files/uploads/HCV_GL_ver6_Dec28_v2.pdf. Accessed 10 Feb 2018.
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996–1005.
Nagaoki Y, Imamura M, Aikata H, et al. The risks of hepatocellular carcinoma development after HCV eradication are similar between patients treated with peg-interferon plus ribavirin and direct-acting antiviral therapy. PLoS One. 2017;12(8):e0182710.
Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and population outcomes of general population screening for hepatitis C. Clin Infect Dis. 2012;54(9):1259–71.
Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156(4):279–90.
McCombs JS, Yuan Y, Shin J, Saab S. Economic burden associated with patients diagnosed with hepatitis C. Clin Ther. 2011;33(9):1268–80.
Manos MM, Darbinian J, Rubin J, et al. The effect of hepatitis C treatment response on medical costs: a longitudinal analysis in an integrated care setting. J Manag Care Pharm. 2013;19(6):438–47.
Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45(2):e17–24.
Backx M, Lewszuk A, White JR, et al. The cost of treatment failure: resource use and costs incurred by hepatitis C virus genotype 1-infected patients who do or do not achieve sustained virological response to therapy. J Viral Hepat. 2014;21(3):208–15.
Acknowledgements
We are grateful to Mr. S. Kondo and Mr. S. Yamakawa from Denno Labo Corporation for their support in extracting the study sample from the NDB.
Author information
Authors and Affiliations
Contributions
HF, YY, DS, SO, SN, RW, and OT contributed to the study’s conception and design; HF carried out the analysis of the data and drafted the manuscript. All authors were involved in the interpretation of the results, as well as in the editing and revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Haruhisa Fukuda, Yoshihiko Yano, Daisuke Sato, Sachiko Ohde, Shinichi Noto, Ryo Watanabe, and Osamu Takahashi have no conflicts of interest, financial or otherwise, to declare.
Funding
This work was supported by a Grant-in-Aid for Health Sciences Research from the Ministry of Health, Labour and Welfare of Japan (Grant number: H30-Seisaku-Shitei-003) and JSPS KAKENHI Grant number 17H04144.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fukuda, H., Yano, Y., Sato, D. et al. Healthcare Expenditures for the Treatment of Patients Infected with Hepatitis C Virus in Japan. PharmacoEconomics 38, 297–306 (2020). https://doi.org/10.1007/s40273-019-00861-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-019-00861-x