Abstract
Background
Clostridium difficile (CD) infection (CDI) causes marked morbidity and mortality, accounting for large healthcare expenditures annually. Current CDI treatment guidelines focus on clinical markers of patient severity to determine the preferred antibiotic regimen of metronidazole versus vancomycin. The antimicrobial resistance patterns for patients with CD are currently unknown.
Aim
The aim of this study was to define the antimicrobial resistance patterns for CD.
Methods
This study included all patients with stools sent for CD testing to a private laboratory (DRG Laboratory, Alpharetta, Georgia) in a 6-month period from across the USA. Patient data was de-identified, with only age, gender, and zip-code available per laboratory protocol. All samples underwent PCR testing followed by hybridization for CD toxin regions A and B. Only patients with CD-positive PCR were analyzed. Antimicrobial resistance testing using stool genomic DNA evaluated presence of imidazole- and vancomycin-resistant genes using multiplex PCR gene detection.
Results
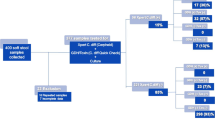
Of 2743, 288 (10.5%) stool samples were positive for CD. Six were excluded per protocol. Of 282, 193 (69.4%) were women, and average age was 49.4 ± 18.7 years. Of 282, 62 were PCR positive for toxins A and B, 160 for toxin A positive alone, and 60 for toxin B positive alone. Antimicrobial resistance testing revealed 134/282 (47.5%) patients resistant to imidazole, 17 (6.1%) resistant to vancomycin, and 9 (3.2%) resistant to imidazole and vancomycin.
Conclusions
CD-positive patients with presence of imidazole-resistant genes from stool DNA extract was a common phenomenon, while vancomycin resistance was uncommon. Similar to treatment of other infections, antimicrobial resistance testing should play a role in CDI clinical decision-making algorithms to enable more expedited and cost-effective delivery of patient care.



Similar content being viewed by others
References
Bartlett JG, Moon N, Chang TW, Taylor N, Onderdonk AB. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology. 1978;75:778–782.
Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478–498.
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145:758–764.
See I, Mu Y, Cohen J, et al. NAP1 strain type predicts outcomes from Clostridium difficile infection. Clin Infect Dis. 2014;58:1394–1400.
Rao K, Micic D, Natarajan M, et al. Clostridium difficile ribotype 027: relationship to age, detectability of toxins A or B in stool with rapid testing, severe infection, and mortality. Clin Infect Dis. 2015;61:233–241.
Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834.
Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis. 2012;55:216–223.
Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2012;34:346–353.
O’Brien JA, Lahue BJ, Caro JJ, Davidson DM. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–1227.
Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;21:431–455.
Debast SB, Bauer MP, Kuijper EJ, European Society of Clinical Microbiology and Infectious Diseases. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect. 2014;20:1–26.
Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. Performance of the xTAG® gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol. 2013;23:1041–1045.
Patel A, Navidad J, Bhattacharyya S. Site-specific clinical evaluation of the Luminex xTAG gastrointestinal pathogen panel for detection of infectious gastroenteritis in fecal specimens. J Clin Microbiol. 2014;52:3068–3071.
EasyMapMaker. (March 2, 2016). Available from: http://www.easymapmaker.com.
Heimesaat MM, Granzow K, Leidinger H, Liesenfeld O. Prevalence of Clostridium difficile toxins A and B and Clostridium perfringens enterotoxin A in stool samples of patients with antibiotic-associated diarrhea. Infection. 2005;33:340–344.
Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–1590.
Johnson S, Louie TJ, Gerding DN, et al. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis. 2014;59:345–354.
Wong SS, Woo PC, Luk WK, Yuen KY. Susceptibility testing of Clostridium difficile against metronidazole and vancomycin by disk diffusion and Etest. Diagn Microbiol Infect Dis. 1999;34:1–6.
Barbut F, Decré D, Burghoffer B, et al. Antimicrobial susceptibilities and serogroups of clinical strains of Clostridium difficile isolated in France in 1991 and 1997. Antimicrob Agents Chemother. 1999;43:2607–2611.
Baines SD, O’Connor R, Freeman J, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother. 2008;62:1046–1052.
Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis. 2011;53:1003–1006.
Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
Funding
The authors report no sources of funding for the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Nimita Fifadara Ph.D. is the Chief Science Officer of DRG Laboratory. Dr. Fifadara played no role in study design, data analysis, or decision to publish. The authors report no other relevant conflict of interest or disclosures.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional Review Board approval was obtained.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Barkin, J.A., Sussman, D.A., Fifadara, N. et al. Clostridium difficile Infection and Patient-Specific Antimicrobial Resistance Testing Reveals a High Metronidazole Resistance Rate. Dig Dis Sci 62, 1035–1042 (2017). https://doi.org/10.1007/s10620-017-4462-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4462-9