Abstract
Background
Esophageal endoscopic submucosal dissection (ESD) has rarely been reported for the treatment of cirrhotic patients.
Aim
To report the results of ESD treatment of superficial esophageal neoplasms (SENs) for cirrhotic patients.
Methods
Forty patients with 50 consecutive SENs undergoing 46 sessions of ESD were retrospectively reviewed. The cirrhotic group included eight patients (11 SENs) with liver cirrhosis consisting of six patients classified as Child-Pugh class A liver cirrhosis and two patients classified as class B liver cirrhosis. Four patients (6 SENs) had coexisting esophageal varices. Parameters were compared between the cirrhotic patients and the non-cirrhotic controls (32 patients, 39 SENs).
Results
Platelet counts of the cirrhotic group were significantly lower, while international normalized ratio was significantly higher. When the cirrhotic group and non-cirrhotic group were compared, the mean tumor length (4 vs. 3.7 cm, p = 0.56) and median procedure time (15.1 vs. 11.5 min/cm2, p = 0.30) were similar. The en bloc resection rates were 81.8 and 89.7 % (p = 0.60). Within the cirrhotic group, both lesions without en bloc resection were patients with esophageal varices. The rates of submucosal disease for the cirrhotic group and non-cirrhotic groups were 54.5 and 25.6 % (p = 0.064), respectively, while the R0 resection rates were 77.8 and 94.3 % (p = 0.16), respectively. The two lesions without R0 resection in cirrhotic group had positive vertical but not horizontal margins due to submucosal invasion. Intraprocedural bleeding occurred more frequently in cirrhotic patients than non-cirrhotic patients (18.2 vs. 0 %, p = 0.045). None of the patients suffered from esophageal perforation, postoperative bleeding, or death that was related to the ESD.
Conclusion
Esophageal ESD seems to be safely and can be effectively performed on cirrhotic patients, particularly those without severe liver dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alcohol and smoking are the two main etiological factors associated with esophageal squamous cell carcinoma (ESCC). This alcohol-smoking combination also predisposes individuals to the occurrence of liver cirrhosis [1]. The rate of liver cirrhosis in patients with esophageal cancer has been reported to be 7 % in a surgical series and 14.3 % in a laparoscopic staging series [2, 3].
Both T1a and T1b ESCC are designated as superficial esophageal neoplasms (SENs) regardless of lymph node or distant organ metastasis [4]. SENs are increasingly detected due to advances in diagnostic techniques and the development of screening programs for high-risk patients [5, 6]. Endoscopic submucosal dissection (ESD) has recently been accepted as a treatment for SENs because it is possible to avoid esophagectomy [7, 8]. However, the technique used for esophageal ESD is very complicated and carries a substantial risk of bleeding (2.1 %) and perforation (5 %) [9]. Occurrence of severe delayed bleeding after ESD is also possible [10]. It seems possible that the use of ESD to treat patients with cirrhosis may carry a higher risk of these complications due to coagulopathy, a reduced platelet count, and/or the presence of coexisting esophageal varices (EVs) [11]. Based on these factors, cirrhotic patients with SENs may have been excluded from ESD treatment by some endoscopists. To our knowledge, the use of esophageal ESD to treat cirrhotic patients has only been reported in one case series [12]. The feasibility of esophageal ESD in cirrhotic patients remains unclear, and how the results obtained treating cirrhotic patients compare with those for non-cirrhotic patients remains unknown. The aims of this retrospective study are to report the efficacy and safety of using ESD to treat SENs present in cirrhotic patients and to compare the outcome of the procedure with that of patients without liver cirrhosis.
Materials and Methods
From January 2012 to July 2015, a total of 40 patients with 50 consecutive SENs underwent 46 sessions of ESD; these were retrospectively identified from a computer database at the Therapeutic Endoscopic Center of Chang Gung Memorial Hospital-Linkou Branch. Twenty-seven patients (67.5 %) had synchronous (n = 21) or metachrnous (n = 6) head and neck cancers with the SENs being diagnosed on routine screening endoscopy. All patients underwent endoscopic ultrasound (EUS) and computed tomography to allow staging before ESD. The patients with biopsy results indicating ESCC also underwent positron emission tomography-computed tomography. The inclusion criteria for ESD were: (1) no lymph node or distant organ metastasis on the pretreatment image studies and (2) those patients with mucosal diseases based on EUS, or those patients with submucosal disease who refused esophagectomy (n = 4, two had liver cirrhosis). The exclusion criteria were that the patient refused to provide inform consent for the ESD.
Among the included patients, eight patients (11 SENs, 10 sessions of ESD) with liver cirrhosis were classified as the cirrhotic group, which was made up of six patients with Child-Pugh class A (CP-A) liver cirrhosis and two patients with Child-Pugh class B (CP-B) liver cirrhosis (Table 1). Liver cirrhosis was diagnosed for all patients based on ultrasonographic findings, the presence of EVs in four patients (6 SENs), and histological proof in three patients (at a previous resection of their hepatomas, 4 SENs). In addition to alcohol as the etiological factor of liver cirrhosis, three patients with hepatomas also had chronic hepatitis B infection. The remaining 32 patients (39 SENs, 36 sessions of ESD) without liver cirrhosis were classified as the non-cirrhotic group. The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (104-0308C).
None of the patients had preoperative ascites or encephalopathy. The patient characteristics of cirrhotic group are listed in Table 1. The SENs of the cirrhotic group were numbered in chronological order according to the ESD procedures performed. Two patients with three (SEN 2, 3, and 5) and two (SEN 8 and 9) SENs underwent three and one endoscopic sessions of ESD, respectively. Before ESD, a fresh-frozen plasma and platelet transfusion were given in one CP-B patient (SEN 6). The shape and size of EVs are presented according to the classification defined by the Japanese Research Society of Portal Hypertension [13]; that is, Form-1 (F-1), F-2, or F-3. The two CP-B patients (SEN 6 and 7) had already received several sessions of endoscopic variceal ligation (EVL) before the ESD. Therefore, the shape and size of the EVs at ESD were F-1 in five procedures (SEN 1, 2, 3, 5, and 7) and F-2 in one procedure (SEN 6). The relationship between SENs and varices was illustrated in Fig. 1a–c. For those patients with coexistent EVs, EVL was performed by starting the application of the bands at the esophagocardiac junction (ECJ) and working upwards in a helical fashion just prior to the ESD in the same endoscopic session using multiband ligators (Cook Medical or Boston Scientific Corp.) for all but one patient (SEN 7). EVL was not performed for the patient with SEN 7 because the circumferential SEN (6 cm in length) involved the ECJ.
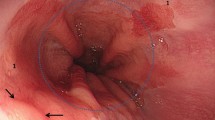
Relationship between esophageal neoplasm and varices (SEN 6). a White light endoscopy showing varices (green arrows) beneath and near to the neoplasm (black arrows). b Endoscopic ultrasound showing a varix (green arrows) beneath the neoplasm in the submucosal layer. c After Lugol’s iodine staining, the varix cannot be visualized
All ESD procedures were performed by a single endoscopist (Tsou YK), and the study started from his first case of esophageal ESD. The endoscopist sought advise from two skilled ESD endoscopists (Ohata K and Fu KI) when there was difficulty with the ESD procedure. The details of the ESD procedure are similar to those described in our previous report [14]. The submucosal injection fluid was a glycerol solution plus indigo carmine with or without epinephrine (0.0004 %). ESD was performed mainly using an insulated tip 2 (IT2) knife (KD-611L, Olympus). En bloc resection was defined as completed target resection in one piece. A complete resection was described as R0 when all the resection margins were free of tumor cells. Procedural complications included major bleeding and perforation. Major bleeding was defined as the necessity for a blood transfusion during the procedure or the presence of post-procedural bleeding requiring endoscopic or surgical hemostasis. Perforation was defined as seeing the structures of the mediastinum endoscopically during the ESD or the detection of free air by plain radiography or computed tomography after the procedure.
Statistical Analysis
The Fisher’s exact test was used to compare categorical variables between the cirrhotic and non-cirrhotic groups. To compare continuous variables of the cirrhotic and non-cirrhotic groups, the Mann–Whitney U test was used for procedure time, while the independent Student’s t test was used for continuous variables other than procedure time. Statistical analyses were performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL). A two-tailed p value of <0.05 was considered statistically significant.
Results
The detailed results of carrying out ESD on the cirrhotic patients are summarized in Table 1. SEN 1 had a disproportionately long procedure time because this was the second esophageal ESD case of the endoscopist. The comparisons of patient and tumor characteristics between the two groups are presented in Table 2. There were no significant differences between the groups with regard to age and gender. In cirrhotic group, the patients’ mean serum level of albumin (3.9 vs. 4.3 g/dL, p = 0.002) and mean platelet count (165.7 vs. 221.1 × 103/mm3, p = 0.027) were significantly lower, while the mean international normalized ratio value (1.23 vs. 1, p = 0.014) was significantly higher than the same values for the non-cirrhotic group. There were three, two, and six SENs located at the upper, middle, and lower third of the esophagus, respectively, in the cirrhotic group. The mean tumor length was similar for the groups (4.0 vs. 3.7 cm, p = 0.56).
None of the patients had ascites or hepatic encephalopathy postoperatively. Table 3 lists the short-term outcomes of the ESD treatment for both groups. The medium procedure time was not statistically different between the groups (15.1 vs. 11.5 min/cm2, p = 0.3). The en bloc resection rate was also similar (9/11 or 81.8 % vs. 35/39 or 89.7 %, p = 0.6). There were two patients in the cirrhotic group who did not undergo en bloc resection, these were SEN 3, which resulted in a piecemeal resection due to the inexperience of the endoscopist and SEN 7 where there was a failure to remove due to the presence of submucosal fibrosis caused by previous repeated EVLs. The R0 resection rate was 7/9 versus 33/35 (77.8 vs. 94.3 %, p = 0.16). The two SENs (SEN 6 and 10) who did not have a R0 resection in the cirrhotic group were positive for vertical but not horizontal margins, because of deep submucosal invasion by the tumors.
Two patients with coexisting EVs in the cirrhotic group (SEN 1 and 7) required a blood transfusion (packed RBC 500 cc) during the ESD procedure (18.2 vs. 0 %, p = 0.045). Both patients received blood transfusions based on preexisting anemia (hemoglobin was 9–10 g/dL), a full suction bottle (1000 cc/bottle), and the prolonged procedure times (327 and 332 min, respectively). Frequent bleeding occurred during both the mucosal incision and the submucosal dissection. Transient spurting that lasted for minutes even occurred during the circumferential submucosal dissection for SEN 7. The inexperience of the endoscopist and the fact that ESDs could be difficult due to submucosal fibrosis were the main cause of the uncontrollable bleeding for SEN1 and SEN7, respectively. However, none of the patients in either group had post-procedural bleeding or esophageal perforation.
The postoperative pathological results and clinical outcomes are listed in Table 1 and Table 3. The depth of tumor invasion was subclassified according to the Japanese Classification of Esophageal Cancer [4]. In the cirrhotic group, two SENs were pT1a-EP (carcinoma in situ), two SENs were pT1a-MM (tumor invading the muscularis mucosa), and another six SENs were pT1b-SM2 (tumor invading the submucosa to a depth of more than 200 μm from the muscularis mucosa). There were six (54.5 %) and ten (25.6 %) SENs with submucosal invasion in cirrhotic and non-cirrhotic groups, respectively (p = 0.064). In cirrhotic group, two patients had a positive vertical resection margin (SEN 6 and 10) and one patient (SEN 9) had microvascular invasion. Among these three patients, one (SEN 10) received surgical treatment and the other two underwent concurrent chemoradiotherapy (CCRT). During a mean follow-up period of 22.6 months (range, 6–30 months), two patients expired. One (SEN 7) died of his synchronous tonsilar cancer at 6 months after the ESD and the other one (SEN 2, 3, and 5) died of pneumonia at 22 months after the ESD. None of the patients in cirrhotic group had tumor recurrence during the follow-up period.
Discussion
From the clinical perception, cirrhotic patients would seem to be poor candidates for invasive procedures such as ESD because of concerns about increased procedure-related complications [11, 15]. However, several studies have reported the feasibility of gastric ESD for patients with liver cirrhosis, reporting post-procedural bleeding rates of 4.3–16.7 % and perforation rates of 0–1.5 % [16–20]. In the present study of esophageal ESD for patients with liver cirrhosis, no patient had post-procedural bleeding or perforation. It has been reported that platelet function or primary hemostasis are not necessarily defective in cirrhotic patients [11, 21]. For elective invasive procedures, only severe thrombocytopenia (<50 × 103/mm3) is used as a cut-off value in terms of contraindications [21]. Our results confirmed that esophageal ESD can be safely performed on cirrhotic patients without severe thrombocytopenia [12].
Regarding esophageal ESD, our previous study revealed that there is a significant learning curve [14]. Therefore, we believed that the two SENs without en bloc resection can have this fact attributed to the inexperience of the endoscopist during the early leaning period. This is supported by the fact that the procedure time was significantly shortened for the later six SENs compared to the earlier five SENs (median 10.9 vs. 24.7 min/cm2, p = 0.006). However, the failure to carry out an en bloc resection with SEN 7 was also due to the presence of esophageal fibrosis caused by previously repeated EVL.
If we consider the en bloc resected specimens, a positive vertical but not horizontal margin may be regarded as tumor factor because of the possibility of deep submucosal invasion, rather than this being attributable to a technique failure of the ESD. In cirrhotic group, two SENs had positive vertical margins but none had a positive horizontal margin. Although there was a high rate (54.5 %) of SENs with submucosal invasion in the cirrhotic group, none of the patients had tumor recurrence during follow-up, including the two patients receiving additional CCRT. Given the fact that esophagectomy in cirrhotic patients is associated with significant morbidity rates of 83–87 % and mortality rates of 17–30 % [22], ESD combined CCRT may be an alternative treatment for cirrhotic patients with submucosal diseases.
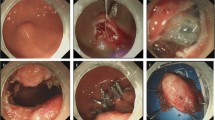
Intraprocedural bleeding with or without the need of blood transfusion did occur more significantly and frequently when the patients had liver cirrhosis, especially among those with visible EVs. This could be due to two reasons. Firstly, despite the fact that we performed the EVL just before the ESD, the varices were still present during the ESD, although their sizes might have been decreased. Eradication of varices usually requires two to four EVL sessions [23]. Therefore, performing several sessions of EVL before ESD, rather than just one session before ESD, may be helpful in minimizing the EVs. Secondly, we used an IT2 knife (Olympus) at specific cutting modes (40 W ENDO-CUT mode with effect 2 using ERBE VIO 200 S or 40 W PULSE-CUT slow mode using Olympus ESG-400) for most of the procedure including both mucosal incision and submucosal dissection. Most of the larger varices could be visualized and pretreated with the 80-W soft coagulation mode using a Coagrasper (Olympus) or using a hot biopsy forceps (Olympus). However, un-visualized or un-noticed smaller vessels were still severed and this resulted in frequent episodes of brisk bleeding compared to the non-cirrhosis patients. In cirrhotic patients without EVs visualized on the preESD endoscopies, smaller varices could still be found during mucosal incision and submucosal dissection (Fig. 2). These frequent episodes of brisk bleeding resulted in a need for frequent hemostatic procedures and increased the difficulty carrying out the ESD; portal hypertension was the presumptive cause [11, 24]. In these circumstances, a combination of portal hypotensive agents and EVL may be an effective method of reducing the severity and frequency of bleeding during ESD. In the later cases, we used the 40 W forced coagulation mode for the submucosal dissection and this also resulted in less severe and frequent bleeding. Further studies are needed to determine the best solution to minimizing intraprocedural bleeding when cirrhotic patients are undergoing esophageal ESD.
The major limitation of the present study is the small case number and the fact that their operations were performed in a single center. However, because of concerns about increased complications, cirrhotic patients with SENs underwent ESD uncommonly and there has previously been only one case series published in the literature [12]. Therefore, we believe that prospective multicenter studies are required to enroll a larger number of patients. The second limitation is that we did not have patients with CP-C cirrhosis or patients with F-3 EVs, and therefore we do not know whether it is feasible for patients with a poor liver function reserve or large EVs to undergo esophageal ESD.
In conclusion, the results of this study indicated that esophageal ESD can be safely and effectively performed in patients with liver cirrhosis by an experienced ESD endoscopist, at least when the patients are without severe liver dysfunction or large EVs.
References
Trivin F, Boucher E, Vauléon E, et al. Management of esophageal carcinoma associated with cirrhosis: a retrospective case-control analysis. J Oncol. 2009;2009:173421–173425.
Tachibana M, Kotoh T, Kinugasa S, et al. Esophageal cancer with cirrhosis of the liver: results of esophagectomy in 18 consecutive patients. Ann Surg Oncol. 2000;7:758–763.
Dagnini G, Caldironi MW, Marin G, Buzzaccarini O, Tremolada C, Ruol A. Laparoscopy in abdominal staging of esophageal carcinoma. Report of 369 cases. Gastrointest Endosc. 1986;32:400–402.
Japanese Classification of Esophageal Cancer, tenth edition: part I. Esophagus. 2009;6:1–25.
Hashimoto CL, Iriya K, Baba ER, et al. Lugol’s dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275–282.
Lee CT, Chang CY, Lee YC, et al. Narrow-band imaging with magnifying endoscopy for the screening of esophageal cancer in patients with primary head and neck cancers. Endoscopy. 2010;42:613–619.
Sun F, Yuan P, Chen T, Hu J. Efficacy and complication of endoscopic submucosal dissection for superficial esophageal carcinoma: a systematic review and meta-analysis. J Cardiothorac Surg. 2014;7:78.
Uno K, Iijima K, Koike T, Shimosegawa T. Useful strategies to prevent severe stricture after endoscopic submucosal dissection for superficial esophageal neoplasm. World J Gastroenterol. 2015;21:7120–7133.
Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862–1869.
Chiba H, Ashikari K, Takahashi A, et al. A case of delayed bleeding after endoscopic submucosal dissection for completely circumferential esophageal cancer. Endoscopy. 2015;47:E385–E386.
Ferro D, Angelico F, Caldwell SH, Violi F. Bleeding and thrombosis in cirrhotic patients: what really matters? Dig Liver Dis. 2012;44:275–279.
Sawaguchi M, Jin M, Matsuhashi T, et al. The feasibility of endoscopic submucosal dissection for superficial esophageal cancer in patients with cirrhosis (with video). Gastrointest Endosc. 2014;79:681–685.
Japanese Research Society for Portal Hypertension (Chairman: Inokuchi K). The general rules for recording endoscopic findings on esophageal varices. Jpn J Surg. 1980;10:84–87.
Tosu YK, Chuang WY, Liu CY, et al. Learning curve for endoscopic submucosal dissection of esophageal neoplasms. Dis Esophagus. 2015. doi:10.1111/dote.12380.
Nicoll A. Surgical risk in patients with cirrhosis. J Gastroenterol Hepatol. 2012;27:1569–1575.
Ogura K, Okamoto M, Sugimoto T, et al. Efficacy and safety of endoscopic submucosal dissection for gastric cancer in patients with liver cirrhosis. Endoscopy. 2008;40:443–445.
Kato M, Nishida T, Hamasaki T, et al. Outcomes of ESD for patients with early gastric cancer and comorbid liver cirrhosis: a propensity score analysis. Surg Endosc. 2015;29:1560–1566.
Kwon YL, Kim ES, Lee KI, et al. Endoscopic treatments of gastric mucosal lesions are not riskier in patients with chronic renal failure or liver cirrhosis. Surg Endosc. 2011;25:1994–1999.
Choi JH, Kim ER, Min B-H, et al. The feasibility and safety of the endoscopic submucosal dissection of superficial gastric neoplastic lesions in patients with compensated liver cirrhosis: a retrospective study. Gut Liver. 2012;6:58–63.
Repici A, Pagano N, Hassan C, et al. Endoscopic submucosal dissection of gastric neoplastic lesions in patients with liver cirrhosis: a systematic review. J Gastrointestin Liver Dis. 2012;21:303–307.
Violi F, Basili S, Raparelli V, Chowdary P, Gatt A, Burroughs AK. Patients with liver cirrhosis suffer from primary haemostatic defects? Fact or fiction? J Hepatol. 2011;55:1415–1427.
Mariette C. Is there a place for esogastric cancer surgery in cirrhotic patients? Ann Surg Oncol. 2008;15:680–682.
Villanueva C, Colomo A, Aracil C, Guarner C. Current endoscopic therapy of variceal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:261–278.
Poza Cordon J, Froilan Torres C, Burgos García A, Gea Rodriguez F, Suárez de Parga JM. Endoscopic management of esophageal varices. World J Gastrointest Endosc. 2012;4:312–322.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Tsou, YK., Liu, CY., Fu, KI. et al. Endoscopic Submucosal Dissection of Superficial Esophageal Neoplasms Is Feasible and Not Riskier for Patients with Liver Cirrhosis. Dig Dis Sci 61, 3565–3571 (2016). https://doi.org/10.1007/s10620-016-4342-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4342-8