Abstract
Background
There is increased awareness about risks and benefits of using domperidone to treat gastroparesis.
Aim
To describe the outcome of treating patients with refractory gastroparesis symptoms with domperidone.
Methods
Domperidone 10 mg QID or TID was prescribed to patients with refractory gastroparesis symptoms; follow-up obtained at 2–3 months assessing symptoms and side effects. Patients filled out Patient Assessment of Upper GI Symptoms prior to treatment and at follow-up along with Clinical Patient Grading Assessment Scale (CPGAS, +7 = completely better; 0 = no change).
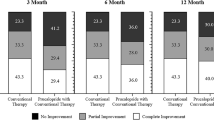
Results
Of 125 patients initially prescribed domperidone, 7 did not take this medication and 3 were lost to follow-up. Of the 115 known patients treated with domperidone, 88 had idiopathic, 16 diabetic, and 9 postsurgical gastroparesis. Side effects were reported by 44 patients (most common—headache, tachycardia/palpitations, diarrhea); 14 patients stopped treatment. Hundred and one patients were seen at follow-up taking domperidone (2.4 ± 2.7 months, average dose 36 ± 13 mg/day). CPGAS averaged 2.7 ± 2.7 (p < 0.01) with 69 patients reporting symptom improvement and 45 patients at least moderately improved with CPGAS ≥ 4. Improvements were seen in most symptoms, especially postprandial fullness, nausea, vomiting, and stomach fullness.
Conclusions
In this large single-center study of patients treated with domperidone, side effects necessitating discontinuing treatment occurred in 12 %. The majority of patients remaining on treatment experienced an improvement in symptoms of gastroparesis, particularly postprandial fullness, nausea, vomiting, and stomach fullness. Thus, domperidone treatment is beneficial for many patients with symptoms of gastroparesis. This study provides needed benefit and risk information concerning treating patients with domperidone. FDA IND Number: 71,089.
Similar content being viewed by others
References
Parkman HP, Hasler WL, Fisher RS, American Gastroenterological Association. American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622.
Abell TL, Bernstein RK, Cutts T, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–283.
Rey E, Choung RS, Schleck CD, Zinsmeister AR, Talley NJ, Locke GR 3rd. Prevalence of hidden gastroparesis in the community: the gastroparesis “iceberg”. J Neurogastroenterol Motil. 2012;18:34–42.
Camilleri M, Parkman HP, Shafi MA, Abell TL, Gerson L, American College of Gastroenterology. Clinical guideline: management of gastroparesis. Am J Gastroenterol. 2013;108:18–37.
Reddymasu SC, Soykan I, McCallum RW. Domperidone: review of pharmacology and clinical applications in gastroenterology. Am J Gastroenterol. 2007;102:2036–2045.
Van Noord C, Dieleman JP, Van Herpen G, et al. Domperidone and ventricular arrhythmia or sudden cardiac death: a population-based case–control study in the Netherlands. Drug Saf. 2010;33:1003–1014.
Johannes CB, Varas-lorenzo C, Mcquay LJ, et al. Risk of serious ventricular arrhythmia and sudden cardiac death in a cohort of users of domperidone: a nested case–control study. Pharmacoepidemiol Drug Saf. 2010;19:881–888.
Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749.
Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–150.
Maranki JL, Lytes V, Meilahn JE, et al. Predictive factors for clinical improvement with Enterra gastric electric stimulation treatment for refractory gastroparesis. Dig Dis Sci. 2008;53:2072–2078.
Simmons K, Parkman HP. Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis. Dig Dis Sci. 2014;59:1231–1234.
Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–1462.
Abell TL, Camilleri M, Donohoe K, et al. Consensus recommendations for gastric emptying scintigraphy. Am J Gastroenterol. 2008;103:753–763.
Patterson D, Abell T, Rothstein R, Koch K, Barnett J. A double-blind multicenter comparison of domperidone and metoclopramide in the treatment of diabetic patients with symptoms of gastroparesis. Am J Gastroenterol. 1999;94:1230–1234.
Silvers D, Kipnes M, Broadstone V, et al. Domperidone in the management of symptoms of diabetic gastroparesis: efficacy, tolerability, and quality-of-life outcomes in a multicenter controlled trial. Clin Ther. 1998;20:438–453.
Domperidone FDA web site. http://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/investigationalnewdrugindapplication/ucm368736.htm.
Sturm A, Holtmann G, Goebell H, Gerken G. Prokinetics in patients with gastroparesis: a systematic analysis. Digestion. 1999;60:422–427.
Sugumar A, Singh A, Pasricha PJ. A systematic review of the efficacy of domperidone for the treatment of diabetic gastroparesis. Clin Gastroenterol Hepatol. 2008;6:726–733.
Doggrell SA, Hancox JC. Cardiac safety concerns for domperidone, an antiemetic and prokinetic, and galactogogue medicine. Expert Opin Drug Saf. 2014;13:131–138.
Arana A, Johannes CB, McQuay LJ, Varas-Lorenzo C, Fife D, Rothman KJ. Risk of out-of-hospital sudden cardiac death in users of domperidone, proton pump inhibitors, or metoclopramide: a population-based nested case–control study. Drug Saf. 2015;38:1187–1199.
Leelakanok N, Holcombe A, Schweizer ML. Domperidone and risk of ventricular arrhythmia and cardiac death: a systematic review and meta-analysis. Clin Drug Investig. 2016;36:97–107.
Ioannou A, Jain A, Kassianos G, Missouris C. Survey of the use of domperidone and the association with QTc prolongation in general practice. Postgrad Med J. 2016;92:390–392.
Buffery PJ, Strother RM. Domperidone safety: a mini-review of the science of QT prolongation and clinical implications of recent global regulatory recommendations. N Z Med J. 2015;128:66–74.
Ortiz A, Cooper CJ, Alvarez A, Gomez Y, Sarosiek I, McCallum RW. Cardiovascular safety profile and clinical experience with high-dose domperidone therapy for nausea and vomiting. Am J Med Sci. 2015;349:421–424.
Youssef AS, Parkman HP, Nagar S. Drug-drug interactions in pharmacologic management of gastroparesis. Neurogastroenterol Motil. 2015;27:1528–1541.
Youssef AS, Argikar UA, Pathikonda M, Parkman HP, Nagar S. Identification of domperidone metabolites in plasma and urine of gastroparesis patients with LC-ESI-MS/MS. Xenobiotica. 2013;43:1073–1083.
Parkman HP, Jacobs MR, Mishra A, et al. Domperidone treatment for gastroparesis: demographic and pharmacogenetic characterization of clinical efficacy and side-effects. Dig Dis Sci. 2011;56:115–124.
Youssef AS, Parkman HP, Nagar S. Domperidone interacts with pioglitazone but not with ondansetron via common CYP metabolism in vitro. Xenobiotica. 2014;44:792–803.
Ung D, Parkman HP, Nagar S. Metabolic interactions between prokinetic agents domperidone and erythromycin: an in vitro analysis. Xenobiotica. 2009;39:749–756.
Authors’ contributions
Ron Schey, MD: study concept and design; interpretation of data critical revision of the manuscript for important intellectual content. Mohammed Saadi, MD: data collection; revision of manuscript for important intellectual content. Deena Midani, MD: data collection; revision of manuscript for important intellectual content. Aaron C. Roberts, BA: data collection; revision of manuscript for important intellectual content. Rahul Parupalli, MD: data collection; revision of manuscript for important intellectual content. Henry P. Parkman, MD: study concept and design; study supervision; analysis and interpretation of data; and drafting and revising of manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No relevant conflict of interest.
Rights and permissions
About this article
Cite this article
Schey, R., Saadi, M., Midani, D. et al. Domperidone to Treat Symptoms of Gastroparesis: Benefits and Side Effects from a Large Single-Center Cohort. Dig Dis Sci 61, 3545–3551 (2016). https://doi.org/10.1007/s10620-016-4272-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4272-5