Abstract
Background and Aims
Adrenomedullin (AM) is a multifunctional biologically active peptide that has an ameliorative effect against inflammatory bowel disease in several experimental models. We reported the first case where AM infusion dramatically improved symptoms and colonoscopy findings in patients with refractory ulcerative colitis (UC). To confirm the reproducibility of the efficacy and safety of AM infusion, this pilot study evaluated the clinical feasibility of intravenous administration of AM in patients with refractory UC.
Methods
Seven patients with active refractory UC participated and received intravenous infusion of AM (1.5 pmol/kg/min) for 8 h daily for 14 days, and their Disease Activity Index (DAI) were evaluated before and 2 and 12 weeks after beginning AM administration.
Results
DAI were improved in all patients after AM administration. Within 2 weeks, marked declines in DAI (≥3 points and ≥30 %) were observed in six patients (85.7 %), while a more modest decline was observed in one patient (14.3 %). Overall mean DAI improved from 9.3 ± 0.6 at baseline to 4.6 ± 0.8 at 2 weeks, and then to 1.2 ± 0.5 at 12 weeks. Endoscopic examination revealed substantial amelioration of ulcers, with mucosal healing and scarring. Four patients remained in clinical remission 12 months after AM treatment. AM administration produced significant increases in plasma AM concentrations (approximately 2.5-fold) that had a mild effect on blood pressure and heart rate, but with no serious adverse effects.
Conclusion
AM is a potentially useful agent that acts via a novel mechanism to safely induce mucosal healing and clinical remission in patients with refractory UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adrenomedullin (AM) is a biologically active peptide first isolated from human pheochromocytoma based on its potent vasodilatory activity [1]. AM is ubiquitously distributed in the body and exerts a wide range of physiological effects in addition to vasodilation, including cardiovascular protection, neovascularization, and suppression of inflammation and apoptosis [2–4]. Moreover, evidence from several studies suggests that AM might be effective clinically as a therapeutic agent for the treatment of cardiovascular diseases such as acute myocardial infarction, heart failure, hypertension, pulmonary hypertension, and limb ischemia [5–8].
AM immunoreactivity has been detected throughout the gastrointestinal tract, with especially high concentrations in the stomach and colon [2]. We previously reported that AM had an ameliorative effect in two experimental colitis models, acetic acid-induced colon ulcer, and dextran sulfate sodium-induced colitis [9, 10]. We further showed that the mechanism of the effect of AM likely involved suppressing the activity of inflammatory cytokines, activating regulatory cytokines in intestinal intra-epithelial lymphocytes, protecting intercellular junctions and exerting antibacterial effects [9]. In addition, AM reportedly exerts beneficial effects on microvascular function and contributes to the re-epithelialization of ulcers in experimental models of colitis [11–13]. Collectively, these findings suggest AM might be an effective agent for the treatment of UC, exerting anti-inflammatory and antibacterial effects and stimulating mucosal regeneration and maintenance of the colonic epithelial barrier.
In addition to experimental models of inflammatory bowel disease (IBD), we previously reported the first case where AM infusion dramatically improved symptoms and colonoscopy findings in patients with refractory UC [14]. To confirm the reproducibility of the efficacy and safety of AM infusion, this pilot study evaluated the clinical feasibility of intravenous administration of AM in patients with refractory UC.
Materials and Methods
Study Design
The present study was a single-center, prospective, nonrandomized clinical study performed between July 2010 and June 2013.
Patient Selection
Eligible patients included males and females who were at least 20 years of age and who had been diagnosed with UC according to the standard criteria for symptoms and standard radiographic and endoscopic criteria. Included in the study were: (1) patients who had moderate-to-severe active UC uncontrollable with 5-aminosalicylic acid (5-ASA) and/or corticosteroid therapy and who could not use calcineurin inhibitors or biological agents for any reason; (2) intractable patients exhibiting steroid resistance; and (3) intractable patients exhibiting steroid dependency. The definitions of steroid resistance and steroid dependency were based on the guidelines for the management of ulcerative colitis in Japan [15]. Steroid resistance was defined as unresponsiveness to an appropriate dose of corticosteroid (a daily dose of 40 mg or more of prednisolone) over at least 7 days. Steroid-dependent patients were defined as those with active UC and who are unable to reduce or be weaned from steroid therapy. Patients were evaluated using the Disease Activity Index (DAI) [16]. As an entry criterion, patients were required to have a total DAI of 6 or higher and a mucosal appearance subscore of 2 or 3.
The exclusion criteria were pregnancy or lactation, fulminant severe UC, a history of malignancy or sign of dysplasia, a history of coronary artery disease or cerebrovascular disease, severe liver or kidney dysfunction, or a history of active infection.
Preparation of Human AM
We prepared human AM as previously described [17]. Briefly, the peptide was chemically synthesized in accordance with current good manufacturing practices (CGMP) at Peptide Institute, Inc, Osaka, Japan. The chemical nature of the peptide and the detailed contents of the vials were verified using reverse-phase high-performance liquid chromatography and amino acid analysis. No measurable endotoxin was detected (<0.01563 EU ml−1), and the material was determined to be pyrogen free by the Japan Food Research Laboratories (Tokyo, Japan). For infusion, AM was dissolved in distilled water with 3.75 % d-mannitol, after which the solution was sterilized by passage through a 0.22-µm filter (Millipore, Co, Billerica, MA).
Treatment
AM was intravenously administrated for 8 h at a rate of 1.5 pmol/kg/min (9 ng/kg/min) daily for 14 days. If a patient’s symptoms worsened at any time, and the investigator decided the study drug could not be continued, the treatment was considered a failure.
Concomitant Medications
While receiving AM, patients were allowed to continue taking drugs containing 5-ASA as long as the dosage of those drugs was not altered during the period starting 2 weeks prior to the beginning of the study. For steroids, the dosage had to remain unchanged during the period of AM administration. The initial steroid dose was 60 mg/day, and it was reduced to 40 mg/day after 2 weeks. Subsequently, the steroid dose was tapered to 5 mg biweekly. The steroid dosage was maintained for the initial 2 weeks in patients who received a dose of prednisolone of 40 mg/day or less. However, patients who were administered a dose of prednisolone ≥60 mg/day were permitted to decrease their dosage during the AM therapy period. Patients who began taking azathioprine or tacrolimus less with 3 months prior to this study were excluded. Patients who began taking azathioprine more than 3 months prior to the start of this study were permitted to continue taking the drug at the same dosage. Treatment with tacrolimus, cyclosporine, or infliximab could not be initiated during the study.
Study Endpoints
The primary endpoint of this study was a clinical response evaluated based on the DAI following the administration of AM. A clinical response was defined as a reduction in total DAI from baseline of at least 3 points and at least 30 %, with an accompanying reduction in the subscore for rectal bleeding of at least 1 point or an absolute subscore for rectal bleeding of 0 or 1. DAI subscores, including endoscopic assessment, were determined within 1 week before AM infusion, 2 weeks after initiating infusion, and then at week 12 after initiating infusion. Secondary endpoints included: clinical remission (defined as a total DAI of 2 points or lower, with no individual subscore exceeding 1 point); endoscopic findings showing mucosal healing (defined as an absolute subscore for endoscopy of 0 or 1); a hemodynamic response to AM administration (tolerability); a difference in the plasma AM concentration before and after AM administration; and the biochemical measurements described below.
Blood Chemistry
Plasma levels of mature AM were measured using a specific immunoassay kit (Shionogi, Osaka, Japan) [18]. Routine hematology and blood chemistry analysis were performed before infusion and 4, 8, and 24 h after the start of infusion. At the same time, plasma concentrations of high-sensitivity C-reactive protein, interleukin-4, interleukin-6, interferon-γ, tumor necrosis factor-α, and transforming growth factor-1β were measured using a commercial laboratory testing service (SRL, Hachioji, Japan).
Statistical Analysis
Statistical analysis was performed using Statview 5.0 (SAS Institute Inc, Cary, NC, USA). The statistical significance of any difference between means was evaluated using a paired t test. Values of p < 0.05 were considered statistically significant.
All authors had access to the study data and had reviewed and approved the final manuscript.
Ethical Considerations
This study was conducted in a manner conforming to the ethical principles of the Declaration of Helsinki. Before beginning this study, we obtained approval from the institutional review board at the participating medical institution. This study was registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN 000003230). All subjects provided written informed consent.
Results
Patient Characteristics
We enrolled seven patients with moderate-to-severe active UC that was refractory to conventional combination therapy. The mean age of the patients was 55.1 ± 10.4 years (mean ± SD; range 37–68 years). The clinical backgrounds of the patients enrolled in this study are summarized in Table 1. Endoscopies performed before the study revealed four patients had left-sided colitis and three had total colitis. All seven patients had first been treated with 5-ASA, while 6 also received steroid therapy and 2 had tried cytapheresis (Table 1). All seven patients were refractory to 5-ASA, and 6 of the 7 patients had steroid-refractory ulcerative colitis. Patient 4 was classified as having steroid-dependent colitis, but the steroid therapy had been discontinued because of a history of excessive steroid dosage. The mean DAI for patients at admission was 10.9 ± 0.4 (mean ± SEM; range 9–12), and the initial therapies did not substantially improve the clinical findings (Table 1). The mean DAI at baseline was 9.3 ± 0.6 (p = 0. 0914 vs. score at admission).
No patient received a calcineurin inhibitor (cyclosporine or tacrolimus) or a biologic (infliximab or adalimumab) if they had a history of tuberculosis or were in an immunocompromised state. One patient (patient 3) refused immunomodulative therapy, but requested treatment with AM.
Efficacy
Clinical Response
A clinical response at week 2, as reflected by the DAI score, was observed in six patients (85.7 %), while one patient (patient 6) (14.3 %) showed a partial response. The mean DAI for all patients significantly improved from 9.3 ± 0.6 at baseline to 4.6 ± 0.8 2 weeks after initiating AM treatment (p < 0.001) (Fig. 1; Table 2). Although patient 4 showed a significant response (reduction of 3 points and 33.3 % from baseline), she failed to induce remission. She required infliximab therapy soon after AM therapy, but that was clinically and endoscopically ineffective. Patient 6 showed a partial response (reduction of 3 points and 27.3 % from baseline), but underwent remission after AM therapy. However, she had a total colectomy to prevent recurrence according to patient’s desire. The other five patients progressed favorably, and their DAI improved to 1.2 ± 0.5 within 12 weeks after initiating treatment (Fig. 1; Table 2). A clinical remission with mucosal healing was observed in one patient (14.3 %) at week 2 and in five patients (71.4 %) at week 12.
In three of four patients examined 1 year after AM treatment (patients 1, 2, and 3), the mucosa of the colon remained in remission without steroid therapy (Table 2). Patient 1 in the present study (Tables 1, 2) was reported previously in a case report [14].
Endoscopic Findings
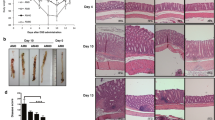
All seven patients were evaluated endoscopically on admission and just before and after AM treatment. Before AM therapy, all seven patients exhibited active inflammatory lesions with diffuse edema, loss of the usual fine vascular pattern, granularity, and easy mucosal bleeding. Furthermore, in six of the patients, wide, deep ulcers were observed in the colon. After 2 weeks of AM therapy, endoscopy showed a reduction in inflammation in all patients, and significant neovascularization and mucosal regeneration were observed at the margins and base of the deep ulcers in those patients who had ulcerative lesions. Of note, remarkable fibroses (scarring) were seen in the remaining relatively shallow ulcers, and scarred regions had a reticulated appearance (Fig. 2). At 12 weeks after initiating AM treatment, these ulcers had disappeared and only clean scars remained (Fig. 2).
Colonoscopic findings. Colonoscopic findings in patients 2, 3, 6, and 7 (representative cases) are shown. Wide and deep ulcers, edema, and erythema were observed in the colon before AM therapy (left panels). Following AM therapy (middle panels), mucosal edema, and erythema were decreased, and significant mucosal regeneration was observed. In patient 7, ulcer scarring was seen. Twelve weeks after initiating AM treatment (right panels), the ulcers had disappeared and ulcer scars were observed. The colonoscopic examination of patient 6 at week 12 was not performed, because she had a total colectomy to prevent recurrence according to her request
Plasma AM Concentration and Serum Biomarkers
Figure 3a shows time-dependent changes in the plasma levels of mature AM during and after AM infusion. The data from patient 4 were omitted because her plasma AM level increased to about 250 fmol/ml (>160 times greater than baseline) on transfusion day 1 and was considered aberrant, as it could not be accounted for by the low dose of exogenous AM administered. Among the remaining six patients, AM levels were unchanged on the day before AM administration, but AM administration produced significant increases in mature AM (approximately 2.5-fold). After stopping AM infusion, plasma AM levels declined to baseline levels by the next morning.
Effect of AM infusion on plasma AM levels. a Plasma AM levels. Symbols depict mean ± SEM. AM infusion was initiated at 0 h and continued for 8 h (horizontal bar at top). *p < 0.05; **p < 0.01 versus baseline. b Blood pressure and pulse rate. Effect of AM infusion on blood pressure and pulse rate. Symbols depict mean ± SEM. AM infusion was initiated at 0 h and was continued for 8 h (horizontal bar at top). Filled circles systolic blood pressure, filled triangles diastolic blood pressure, open diamonds heart rate. *p < 0.05 versus baseline
Serum interleukin-6 and tumor necrosis factor-α levels tended to be lower after 8 h of AM infusion than before infusion (interleukin-6: 12.0 ± 14.1 vs. 6.6 ± 9.0 pg/ml, p = 0.069; tumor necrosis factor-α: 1.5 ± 0.8 vs. 1.2 ± 0.7 pg/ml, p = 0.17). AM infusion was not associated with a change in any other serum biomarkers (high-sensitivity C-reactive protein, interleukin-4, interferon-γ, and transforming growth factor-1β; data not shown).
Safety
AM therapy was generally well tolerated by all seven patients. No severe adverse events were observed during or after AM infusion (Table 3). Patient 4 developed a transient high fever about 4 h after completion of the first AM administration that declined in one night, which was suspected of being caused by a temporary infection.
Changes in blood pressure and heart rate during AM infusion are shown in Fig. 3b. The primary biological effects of AM are a reduction in blood pressure and an increase in heart rate, but these effects were mild in our patients and did not cause clinical symptoms such as fainting or headache. Blood pressure and heart rate returned to their baseline levels soon after the AM infusion finished. Additionally, no significant liver or renal dysfunction was observed during or after AM administration.
Discussion
We evaluated the safety and efficacy of exogenous AM administered to patients with intractable UC in a preliminary open-label exploratory clinical study. Although the number of patients enrolled was small, AM therapy might be useful to ameliorate otherwise intractable UC, as it stimulated mucosal regeneration accompanied by neovascularization and vasodilation visible by endoscopic examination, without significant adverse effects. We previously reported the first case where AM infusion dramatically improved symptoms and colonoscopy findings in patients with refractory UC [14]. The present clinical study clearly shows the reproducibility of AM therapy in intractable UC.
In the present study, all patients experienced some improvement of their clinical symptoms with the administration of AM, and over 80 % (6 of 7) showed a clinical response in DAI within 2 weeks (Table 2). Patients 4 and 6 did not achieve a full clinical response; however, patient 6 showed favorable remission but underwent a total colectomy to prevent recurrence in accordance with her wishes. It is noteworthy that the endoscopic findings of patient 4 showed diffuse edematous and coarse granular mucosa without distinct ulcers, which are target of AM therapy.
Although the mechanisms of action of AM against colitis is still unclear, earlier observations by others and ourselves using experimental colitis models suggest several possible mechanisms [9–13, 19]. AM exerts beneficial effects against experimental colitis by regulating inflammatory responses, for example, the suppression of systemic and local production of inflammatory cytokines. It was also shown that AM contributed to mucosal protection and regeneration, the re-epithelialization of injured mucosal tissue, and the maintenance of epithelial intercellular junctions [9]. In addition, AM stimulated angiogenesis and improved microcirculation and mucosal permeability [9]. In the present study, endoscopic findings showed significant regeneration of the mucosa with scarring and marked angiogenesis, suggesting mucosal healing. AM thus appears to have multiple beneficial effects against colitis, suggesting its therapeutic mechanism may be complex.
In the acetic acid-induced colonic ulcer model [10], a solitary ulcer is induced by the subserosal injection of acetic acid into the colon. Thus, ulceration is the result of a chemical injury rather than an immunological disorder, such as that seen in the dextran sulfate sodium- or 2,4,6-trinitrobenzenesulfonic acid-induced colitis models. AM was effective in all three models, which is consistent with the idea that it acts not only by inhibiting the production of inflammatory cytokines, but also by stimulating tissue reconstruction through re-epithelialization and vascularization.
Following the development of biologics, which are highly effective for the treatment of IBD, mucosal healing by endoscopy is considered an important indicator of efficacy, and mucosal healing with the extinction of acute inflammation is now established as the therapeutic goal in IBD [20]. Collectively, our findings suggest that in patients with colitis, AM may exert both anti-inflammatory effects and effects contributing to mucosal regeneration with vascularization, which indicate AM is a potential candidate for use in the treatment of IBD.
Because AM is a biologically active peptide with potent hypotensive activity, continuous intravenous infusion of AM should be employed. We used a relatively low dose of AM (1.5 pmol/kg/min), and it was infused for only 8 h daily for 14 days. We previously characterized the hemodynamic and hormonal effects of exogenous AM in humans and showed that prolonged administration elicited a strong and steady reduction in blood pressure with significant increases in the plasma AM concentration [17, 21]. In patients with essential hypertension, for example, continuous AM infusion at 2.5 pmol/kg/min (15 ng/kg/min) for 27 h safely caused a significant reduction in blood pressure. Kataoka et al. [6] reported a pilot study examining the effects of intravenous AM administration in patients with acute myocardial infarction. They found that AM infusion at 12.5–25 ng/kg/min for 12 h significantly improved cardiac wall motion in the infarct area, while the hemodynamic parameters remained nearly unchanged. We selected an AM dose of 1.5 pmol/kg/min (9 ng/kg/min) for 8 h, which produced about a 2.5-fold increase in plasma AM levels during infusion (Fig. 3a). This was accompanied by mild changes in blood pressure and heart rate with no adverse effects such as headache or fainting (Fig. 3b). Although the most serious side effect of AM is hypotension, the present study demonstrated the safety of intravenous AM infusion in patients with refractory ulcerative colitis in regard to the threat of developing hypotension.
The therapeutic effects of AM against experimental colitis [12, 13, 22] and gastritis [23] exhibited a bell-shaped dose–response; that is, the beneficial effects of AM increased as the dose increased until a peak was reached, after which the benefits were reduced as the dose was increased further. Thus, although the AM dose used in this study showed both efficacy and safety, the optimal dose remains unknown.
Patients suitable for AM therapy are limited because they must stay in hospital for more than 2 weeks. We mainly enrolled patients with moderate-to-severe refractory UC who were unable to take anything by mouth. Furthermore, patients enrolled in the present study were unable to receive immunosuppressants or biologics because of concern about their age and/or underlying diseases, which included impaired glucose tolerance, renal insufficiency, cardiovascular disease, and past tuberculosis.
It was shown in patients with intractable moderate-to-severe active UC that a poor response to high-dose steroid therapy is often treatable with immunosuppressants (e.g., cyclosporine or tacrolimus) or biologics (e.g., infliximab or adalimumab) [24]. Although they are generally effective, a considerable number of patients respond poorly to these agents, or the drugs show a progressive loss of efficacy [25]. Because AM is an endogenous biologically active peptide in human, antibodies against AM administered should not be produced. Therefore, AM might form the basis of a safe medicinal drug without anaphylactic reaction or progressive loss of efficacy.
The limitations of this study include its open-label, nonrandomized, single-arm, single-dose design, its small sample size, and the possible subjectivity of the DAI scoring. In addition, because of the strict inclusion criteria, only a small number of patients were eligible for this study, which was a limitation of this single-center clinical study.
In summary, we have shown that continuous intravenous infusion of AM at a dose of 1.5 pmol/kg/min (9 ng/kg/min) produced beneficial effects that ameliorated the symptoms of intractable UC without adverse side effects. Moreover, endoscopic evaluation after AM therapy revealed remarkable mucosal regeneration and healing with neovascularization at previously ulcerative or erosive lesions. These results suggest AM is a potentially useful therapeutic agent with a novel mechanism of action involving anti-inflammatory effects with mucosal and vascular regeneration. We are now preparing for an investigator initiated clinical trial of an AM formulation to develop a therapeutic pharmaceutical product for refractory UC.
References
Kitamura K, Kangawa K, Kawamoto M, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560.
Asada Y, Hara S, Marutsuka K, et al. Novel distribution of adrenomedullin-immunoreactive cells in human tissues. Histochem Cell Biol. 1999;112:185–191.
Cheung BM, Tang F. Adrenomedullin: exciting new horizons. Recent Pat Endocr Metab Immune Drug Discov. 2012;6:4–17.
Minamino N, Kikumoto K, Isumi Y. Regulation of adrenomedullin expression and release. Microsc Res Tech. 2002;57:28–39.
Iwase T, Nagaya N, Fujii T, et al. Adrenomedullin enhances angiogenic potency of bone marrow transplantation in a rat model of hindlimb ischemia. Circulation. 2005;111:356–362.
Kataoka Y, Miyazaki S, Yasuda S, et al. The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J Cardiovasc Pharmacol. 2010;56:413–419.
Nagaya N, Nishikimi T, Uematsu M, et al. Haemodynamic and hormonal effects of adrenomedullin in patients with pulmonary hypertension. Heart. 2000;84:653–658.
Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101:498–503.
Ashizuka S, Inagaki-Ohara K, Kuwasako K, Kato J, Inatsu H, Kitamura K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol. 2009;53:573–581.
Ashizuka S, Ishikawa N, Kato J, et al. Effect of adrenomedullin administration on acetic acid-induced colitis in rats. Peptides. 2005;26:2610–2615.
Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Therapeutic effect of urocortin and adrenomedullin in a murine model of Crohn’s disease. Gut. 2006;55:824–832.
Hayashi Y, Narumi K, Tsuji S, et al. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int J Colorectal Dis. 2011;26:1453–1462.
Talero E, de Sotomayor MA, Sanchez-Fidalgo S, Motilva V. Vascular contribution of adrenomedullin to microcirculatory improvement in experimental colitis. Eur J Pharmacol. 2011;670:601–607.
Ashizuka S, Kita T, Inatsu H, Kitamura K. Adrenomedullin: a novel therapy for intractable ulcerative colitis. Inflamm Bowel Dis. 2013;19:E26–E27.
Hibi T, Fumiaki U, Katsuyoshi M, Cheng LT. Guidelines for the Management of Ulcerative Colitis in Japan – Developed through Integration of Evidence and Consensus among Experts. IBD Res. 2010;4:189–239.
Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629.
Kita T, Suzuki Y, Kitamura K. Hemodynamic and hormonal effects of exogenous adrenomedullin administration in humans and relationship to insulin resistance. Hypertens Res. 2010;33:314–319.
Ohta H, Tsuji T, Asai S, et al. One-step direct assay for mature-type adrenomedullin with monoclonal antibodies Clin Chem.. 1999;45:244–251.
Talero E, Sanchez-Fidalgo S, de la Lastra CA, Illanes M, Calvo JR, Motilva V. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides. 2008;29:2001–2012.
Gisbert JP, Chaparro M. Systematic review with meta-analysis: inflammatory bowel disease in the elderly. Aliment Pharmacol Ther. 2014;39:459–477.
Kita T, Tokashiki M, Kitamura K. Aldosterone antisecretagogue and antihypertensive actions of adrenomedullin in patients with primary aldosteronism. Hypertens Res. 2010;33:374–379.
Ashizuka S, Inatsu H, Inagaki-Ohara K, Kita T, Kitamura K. Adrenomedullin as a potential therapeutic agent for inflammatory bowel disease. Curr Protein Pept Sci. 2013;14:246–255.
Clementi G, Caruso A, Cutuli VM, et al. Gastroprotective effect of adrenomedullin administered subcutaneously in the rat. Peptides. 2002;23:1149–1153.
Burger D, Travis S. Conventional medical management of inflammatory bowel disease. Gastroenterology. 2011;140:1827–1837. e2.
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106:685–698.
Acknowledgments
The authors thank Ms. Mariko Tokashiki for measuring adrenomedullin and Dr. Koji Nakashima for helpful discussions. This study was supported in part by a Health and Labor Science Research Grant for Translational Research from the Ministry of Health, Labour and Welfare, Japan; a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS), Japan; a Specific Research Grant from the Uehara Memorial Foundation, Japan; a Grant-in-Aid for Vascular Biology Innovation from the Japan Foundation for Applied Enzymology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Shinya Ashizuka and Haruhiko Inatsu shared co-first authorship.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ashizuka, S., Inatsu, H., Kita, T. et al. Adrenomedullin Therapy in Patients with Refractory Ulcerative Colitis: A Case Series. Dig Dis Sci 61, 872–880 (2016). https://doi.org/10.1007/s10620-015-3917-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3917-0