Abstract
Backgrounds and Aims
We studied the intestinotrophic hormone glucagon-like peptide-2 (GLP-2) as a possible therapy for non-steroidal anti-inflammatory drug (NSAID)-induced intestinal ulcers. Luminal nutrients release endogenous GLP-2 from enteroendocrine L cells. Since GLP-2 is degraded by dipeptidyl peptidase IV (DPPIV), we hypothesized that DPPIV inhibition combined with luminal administration of nutrients potentiates the effects of endogenous GLP-2 on intestinal injury.
Methods
Intestinal injury was induced by indomethacin (10 mg/kg, sc) in fed rats. The long-acting DPPIV inhibitor K579 was given intragastrically (ig) or intraperitoneally (ip) before or after indomethacin treatment. l-Alanine (l-Ala) and inosine 5′-monophosphate (IMP) were co-administered ig after the treatment.
Results
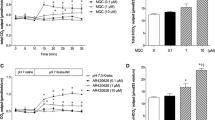
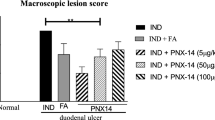
Indomethacin treatment induced intestinal ulcers that gradually healed after treatment. Pretreatment with ig or ip K579 given at 1 mg/kg reduced total ulcer length, whereas K579 at 3 mg/kg had no effect. Exogenous GLP-2 also reduced intestinal ulcers. The preventive effect of K579 was dose-dependently inhibited by a GLP-2 receptor antagonist. Daily treatment with K579 (1 mg/kg), GLP-2, or l-Ala + IMP after indomethacin treatment reduced total ulcer length. Co-administration (ig) of K579 and l-Ala + IMP further accelerated intestinal ulcer healing.
Conclusion
DPPIV inhibition and exogenous GLP-2 prevented the formation and promoted the healing of indomethacin-induced intestinal ulcers, although high-dose DPPIV inhibition reversed the preventive effect. Umami receptor agonists also enhanced the healing effects of the DPPIV inhibitor. The combination of DPPIV inhibition and luminal nutrient-induced GLP-2 release may be a useful therapeutic tool for the treatment of NSAIDs-induced intestinal ulcers.







Similar content being viewed by others
Abbreviations
- DPPIV:
-
Dipeptidyl peptidase-IV
- IMP:
-
Inosine 5′-monophosphate
- IND:
-
Indomethacin
- l-Ala:
-
l-alanine
- l-Glu:
-
l-glutamate
- GLP:
-
Glucagon-like peptide
- GLP-2R:
-
GLP-2 receptor
- PV:
-
Portal venous
- T1R:
-
Taste receptor type 1
References
Higuchi K, Umegaki E, Watanabe T, et al. Present status and strategy of NSAIDs-induced small bowel injury. J Gastroenterol. 2009;44:879–888.
Matsumoto T, Kudo T, Esaki M, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496.
Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59.
Bjarnason I, Hayllar J, Smethurst P, Price A, Gumpel MJ. Metronidazole reduces intestinal inflammation and blood loss in non-steroidal anti-inflammatory drug induced enteropathy. Gut. 1992;33:1204–1208.
Morris AJ, Murray L, Sturrock RD, Madhok R, Capell HA, Mackenzie JF. Short report: the effect of misoprostol on the anaemia of NSAID enteropathy. Aliment Pharmacol Ther. 1994;8:343–346.
Hayllar J, Smith T, Macpherson A, Price AB, Gumpel M, Bjarnason I. Nonsteroidal antiinflammatory drug-induced small intestinal inflammation and blood loss. Effects of sulfasalazine and other disease-modifying antirheumatic drugs. Arthritis Rheum. 1994;37:1146–1150.
Drucker DJ. Glucagon-like peptide 2. J Clin Endocrinol Metab. 2010;10:153–156.
Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964–G972.
Drucker DJ, Yusta B, Boushey RP, DeForest L, Brubaker PL. Human [Gly2]GLP-2 reduces the severity of colonic injury in a murine model of experimental colitis. Am J Physiol. 1999;276:G79–G91.
Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914.
Buchman AL, Katz S, Fang JC, Bernstein CN, Abou-Assi SG. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn’s disease. Inflamm Bowel Dis. 2010;16:962–973.
Boushey RP, Yusta B, Drucker DJ. Glucagon-like peptide 2 decreases mortality and reduces the severity of indomethacin-induced murine enteritis. Am J Physiol. 1999;277:E937–E947.
Rowland KJ, Trivedi S, Lee D, et al. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141:2166–2175.
Hansen L, Hare KJ, Hartmann B, et al. Metabolism of glucagon-like peptide-2 in pigs: role of dipeptidyl peptidase IV. Regul Pept. 2007;138:126–132.
Hartmann B, Thulesen J, Kissow H, et al. Dipeptidyl peptidase IV inhibition enhances the intestinotrophic effect of glucagon-like peptide-2 in rats and mice. Endocrinology. 2000;141:4013–4020.
Okawada M, Holst JJ, Teitelbaum DH. Administration of a dipeptidyl peptidase IV inhibitor enhances the intestinal adaptation in a mouse model of short bowel syndrome. Surgery. 2011;150:217–223.
Engelstoft MS, Egerod KL, Holst B, Schwartz TW. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–449.
Akiba Y, Watanabe C, Mizumori M, Kaunitz JD. Luminal l-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physiol Gastrointest Liver Physiol. 2009;297:G781–G791.
Wang JH, Inoue T, Higashiyama M, et al. Umami receptor activation increases duodenal bicarbonate secretion via glucagon-like peptide-2 release in rats. J Pharmacol Exp Ther. 2011;339:464–473.
Inoue T, Wang JH, Higashiyama M, et al. Dipeptidyl peptidase IV inhibition potentiates amino acid- and bile acid-induced bicarbonate secretion in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2012;303:G810–G816.
Satoh H, Guth PH, Grossman MI. Role of food in gastrointestinal ulceration produced by Indomethacin in the Rat. Gastroenterology. 1982;83:210–215.
Hatazawa R, Ohno R, Tanigami M, Tanaka A, Takeuchi K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J Pharmacol Exp Ther. 2006;318:691–699.
Iwai T, Ichikawa T, Kida M, et al. Vulnerable sites and changes in mucin in the rat small intestine after non-steroidal anti-inflammatory drugs administration. Dig Dis Sci. 2010;55:3369–3376.
Takasaki K, Iwase M, Nakajima T, et al. K579, a slow-binding inhibitor of dipeptidyl peptidase IV, is a long-acting hypoglycemic agent. Eur J Pharmacol. 2004;486:335–342.
Takasaki K, Takada H, Nakajima T, Ueno K, Ushiki J, Higo K. Involvement of the active metabolites in the inhibitory activity of K579 on rat plasma dipeptidyl peptidase IV. Eur J Pharmacol. 2004;505:237–241.
Lambeir AM, Durinx C, Scharpe S, De M. I: dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40:209–294.
Scott RB, Kirk D, MacNaughton WK, Meddings JB. GLP-2 augments the adaptive response to massive intestinal resection in rat. Am J Physiol. 1998;275:G911–G921.
Torres S, Thim L, Milliat F, et al. Glucagon-like peptide-2 improves both acute and late experimental radiation enteritis in the rat. Int J Radiat Oncol Biol Phys. 2007;69:1563–1571.
Moore BA, Peffer N, Pirone A, et al. GLP-2 receptor agonism ameliorates inflammation and gastrointestinal stasis in murine postoperative ileus. J Pharmacol Exp Ther. 2010;333:574–583.
Shin ED, Estall JL, Izzo A, Drucker DJ, Brubaker PL. Mucosal adaptation to enteral nutrients is dependent on the physiologic actions of glucagon-like peptide-2 in mice. Gastroenterology. 2005;128:1340–1353.
Nelson DW, Murali SG, Liu X, Koopmann MC, Holst JJ, Ney DM. Insulin-like growth factor I and glucagon-like peptide-2 responses to fasting followed by controlled or ad libitum refeeding in rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1175–R1184.
Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology. 2010;138:2447–2456.
Takeuchi K, Kita K, Hayashi S, Aihara E. Regulatory mechanism of duodenal bicarbonate secretion: roles of endogenous prostaglandins and nitric oxide. Pharmacol Ther. 2011;130:59–70.
Akiba Y, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Dynamic regulation of mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol. 2000;279:G437–G447.
Takeuchi K, Miyazawa T, Tanaka A, Kato S, Kunikata T. Pathogenic importance of intestinal hypermotility in NSAID-induced small intestinal damage in rats. Digestion. 2002;66:30–41.
Omatsu T, Naito Y, Handa O, et al. Reactive oxygen species-quenching and anti-apoptotic effect of polaprezinc on indomethacin-induced small intestinal epithelial cell injury. J Gastroenterol. 2010;45:692–702.
Hawkey CJ, Karrasch JA, Szczepanski L, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus Misoprostol for NSAID-induced Ulcer Management (OMNIUM) Study Group. New Engl J Med. 1998;338:727–734.
Yoda Y, Amagase K, Kato S, et al. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin -induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61:287–294.
Kato S, Tanaka A, Kunikata T, Umeda M, Takeuchi K. Protective effect of lafutidine against indomethacin-induced intestinal ulceration in rats: relation to capsaicin-sensitive sensory neurons. Digestion. 2000;61:39–46.
Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322.
Herman GA, Stevens C, Van DK, et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin Pharmacol Ther. 2005;78:675–688.
Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164.
Shimizu K, Koga H, Iida M, Haruma K. Microcirculatory changes in experimental mesenteric longitudinal ulcers of the small intestine in rats. Dig Dis Sci. 2007;52:3019–3028.
Bozkurt A, Naslund E, Holst JJ, Hellstrom PM. GLP-1 and GLP-2 act in concert to inhibit fasted, but not fed, small bowel motility in the rat. Regul Pept. 2002;107:129–135.
Amato A, Baldassano S, Serio R, Mule F. Glucagon-like peptide-2 relaxes mouse stomach through vasoactive intestinal peptide release. Am J Physiol Gastrointest Liver Physiol. 2009;296:G678–G684.
Lee SJ, Lee J, Li KK, et al. Disruption of the murine Glp2r impairs Paneth cell function and increases susceptibility to small bowel enteritis. Endocrinology. 2012;153:1141–1151.
Drucker DJ, Erlich P, Asa SL, Brubaker PL. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci USA. 1996;93:7911–7916.
Xiao Q, Boushey RP, Cino M, Drucker DJ, Brubaker PL. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1057–R1063.
Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140:5356–5363.
Amagase K, Ochi A, Kojo A, et al. New therapeutic strategy for amino acid medicine: prophylactic and healing promoting effect of monosodium glutamate against NSAID-induced enteropathy. J Pharmacol Sci. 2012;118:131–137.
Yamazaki K, Yasuda N, Inoue T, et al. The combination of metformin and a dipeptidyl peptidase IV inhibitor prevents 5-fluorouracil-induced reduction of small intestine weight. Eur J Pharmacol. 2004;488:213–218.
Liu X, Nelson DW, Holst JJ, Ney DM. Synergistic effect of supplemental enteral nutrients and exogenous glucagon-like peptide 2 on intestinal adaptation in a rat model of short bowel syndrome. Am J Clin Nutr. 2006;84:1142–1150.
Liu X, Murali SG, Holst JJ, Ney DM. Enteral nutrients potentiate the intestinotrophic action of glucagon-like peptide-2 in association with increased insulin-like growth factor-I responses in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1794–R1802.
Acknowledgments
We thank Coleen and Bea Palileo for their assistance with manuscript preparation. Supported by the Department of Veterans Affairs Merit Review Award, NIH-NIDDK R01 DK54221 (J. Kaunitz), and the animal core of NIH-NIDDK P30 DK0413 (J.E. Rozengurt).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, T., Higashiyama, M., Kaji, I. et al. Dipeptidyl Peptidase IV Inhibition Prevents the Formation and Promotes the Healing of Indomethacin-Induced Intestinal Ulcers in Rats. Dig Dis Sci 59, 1286–1295 (2014). https://doi.org/10.1007/s10620-013-3001-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-3001-6