Abstract
Background
De-regulation of Wnt signalling is increasingly being implicated in both experimental and human carcinogenesis including colon cancer.
Aims
Our goal was to identify possible dietary agents that block Wnt signalling as a step toward investigating new strategies for suppression of colon cancer. Pomegranate extract has emerged as an intriguing candidate due to its polyphenolic content.
Methods
We used a 1,2-dimethylhydrazine dihydrochloride (DMH)-induced rat colon carcinogenesis model to investigate the expression pattern of the main key players in Wnt signalling by reverse transcription polymerase chain reaction (RT-PCR) analysis.
Results
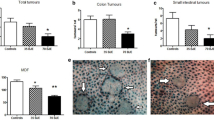
Our results showed that many Wnt-target genes, e.g., Wnt5a, frizzled receptor (FRZ)-8, β-catenin, T cell factor/lymphoid enhancer binding protein (Tcf4/Lef1), c-myc and cyclin D1, were up-regulated whereas adenomatous polyposis coli (APC) and axin1 exhibited down-regulation in colonic tissues of our DMH-colon cancer group compared with the normal group. Standardized pomegranate extract minimised all the aberrant alterations observed in the studied Wnt genes in colonic tissues of the DMH + pomegranate group as compared with the DMH-induced colon cancer group. This effect was also confirmed by the normalization of survival rate, inhibition of tumour incidence and a reduction of serum tumour marker carcinoembryonic antigen (CEA) level. Histopathological observations provided supportive evidence for the biochemical and molecular analyses.
Conclusions
Standardized pomegranate extract holds great promise in the field of colon cancer prevention by dietary agents.




Similar content being viewed by others
Abbreviations
- APC:
-
Adenomatous polyposis coli
- AOM:
-
Azoxymethane
- CK2:
-
Casein kinase2
- CEA:
-
Carcinoembryonic antigen
- DMH:
-
1,2-dimethylhydrazine
- EA:
-
Ellagic acid
- FRZ:
-
Frizzled receptors
- GAEs:
-
Gallic acid equivalents
- GSK3β:
-
Glycogen synthase kinase 3β
- JNKs:
-
c-Jun N-terminal kinase
- PKCa:
-
Protein kinase Ca
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- Tcf/Lef:
-
T cell factor/lymphoid enhancer binding protein
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. 2009. CA Cancer J Clin. 2009;59:225–249.
LaMont JT, O’Gorman TA. Experimental colon cancer. Gastroenterology. 1978;75:1157–1169.
Femia AP, Caderni G. Rodent models of colon carcinogenesis for the study of chemopreventive activity of natural products. Planta Med. 2008;74:1602–1607.
Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398.
Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162.
Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305.
Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850.
Kawano Y, Kypta R. Secreted antagonists of the Wnt signaling pathway. J Cell Sci. 2003;116:2627–2634.
Zorn AM. Wnt signalling: antagonistic Dickkopfs. Curr Biol. 2001;11:R592–R595.
Hernández-Maqueda JG, Luna-Ulloa LB, Santoyo-Ramos P, Castañeda-Patlán MC, Robles-Flores M. Protein kinase C delta negatively modulates canonical Wnt pathway and cell proliferation in colon tumor cell lines. PLoS ONE. 2013;8:e58540.
Clements WM, Lowy AM, Groden J. Adenomatous polyposis coli/beta-catenin interaction and downstream targets: altered gene expression in gastrointestinal tumors. Clin Colorectal Cancer. 2003;3:113–120.
Macleod RJ. Extracellular calcium-sensing receptor/PTH knockout mice colons have increased Wnt/β-catenin signaling, reduced non-canonical Wnt signaling, and increased susceptibility to azoxymethane-induced aberrant crypt foci. Lab Invest. 2013;1:520–527.
Corpet DE, Pierre F. How good are rodent models of carcinogenesis in predicting efficacy in humans? A systematic review and meta-analysis of colon chemoprevention in rats, mice and men. Eur J Cancer. 2005;4:1911–9922.
van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European prospective investigation into cancer and nutrition. Am J Clin Nutr. 2009;89:1441–1452.
Zaid H, Silbermann M, Ben-Arye E, Saad B. Greco-arab and Islamic herbal-derived anticancer modalities: from tradition to molecular mechanisms. Evid Based Complement Alternat Med. 2012;2012:349040. doi:10.1155/2012/349040.
Lansky EP, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206.
Mertens-Talcott SU, Jilma-Stohlawetz P, Rios J, Hingorani L, Derendorf H. Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum L.) polyphenols after ingestion of a standardized extract in healthy human volunteers. J Agric Food Chem. 2006;54:8956–8961.
Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antiox Redox Signal. 2008;10:475–510.
Waly MI, Ali A, Guizani N, Al-Rawahi AS, Farooq SA, Rahman MS. Pomegranate (Punica granatum) peel extract efficacy as a dietary antioxidant against azoxymethane-induced colon cancer in rat. Asian Pac J Cancer Prev. 2012;13:4051–4055.
Syed DN, Chamcheu JC, Mukhtar VM. Pomegranate extracts and cancer prevention: molecular and cellular activities. Anticancer Agents Med Chem. 2012 Oct 12. (Epub ahead of print).
Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: a review of potential anti-inflammatory and anti-infective effects. J Ethnopharmacol. 2012;143:397–405.
Neyrinck AM, Van Hée VF, Bindels LB, De Backer F, Cani PD, Delzenne NM. Polyphenol-rich extract of pomegranate peel alleviates tissue inflammation and hypercholesterolaemia in high-fat diet-induced obese mice: potential implication of the gut microbiota. Br J Nutr. 2013;109:802–809.
Gil MI, Tomas-Barberan FA, Hess-Pierce B, Holcroft DM, Kader AA. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agric Food Chem. 2000;48:4581–4589.
Seeram NP, Adams LS, Henning SM, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367.
Heber D. Pomegranate ellagitannins. In: Benzie IFF, Wachtel-Galor S, eds. Herbal Medicine: Biomolecular and Clinical Aspects, 2nd edition, Chapter 10. Boca Raton (FL): CRC Press; 2011.
Larrosa M, Tomas-Barberan FA, Espin JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J Nutr Biochem. 2006;17:611–625.
Seeram NP, Lee R, Hardy ML, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a byproduct of the commercial juice industry. Sep Purif Technol. 2005;41:49–55.
Seeram NP, Zhang Y, McKeever R, et al. Pomegranate juice and extracts provide similar levels of plasma and urinary ellagitannin metabolites in human subjects. J Med Food. 2008;11:390–394.
Hope C, Planutis K, Planutiene M, et al. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52:S52–S61.
Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-κB-signaling pathways. Carcinogenesis. 2009;30:300–307.
Pacheco-Palencia LA, Noratto G, Hingorani L, Talcott ST, Mertens-Talcott SU. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet irradiated human skin fibroblasts. J Agric Food Chem. 2008;56:8434–8441.
Singleton VL, Esau P. Phenolic substances in grapes and wine, and their significance. Adv Food Res. 1969; Suppl. 1:1–261.
Sharma P, Kaur J, Sanyal SN. Effect of etoricoxib, a cyclooxygenase-2 selective inhibitor on aberrant crypt formation and apoptosis in 1,2 dimethyl hydrazine induced colon carcinogenesis in rat model. Nutr Hosp. 2010;25:39–48.
Hossin FLA. Effect of pomegranate (Punica granatum) peels and it’s extract on obese hypercholesterolemic rats. Pak J Nutr. 2009;8:1251–1257.
Chomkczynski P, Sacchi N. Single step method of RNA isolation by acid guanidinium thiocyanate-phenolchloroform method. Anal Biochem. 1987;162:156–160.
Daudet N, Ripoll C, Molès JP, Rebillard G. Expression of members of Wnt and Frizzled gene families in the postnatal rat cochlea. Brain Res Mol Brain Res. 2002;105:98–107.
Wang QM, Zhang Y, Yang KM, Zhou HY, Yang HJ. Wnt/beta-catenin signaling pathway is active in pancreatic development of rat embryo. World J Gastroenterol. 2006;28:2615–2619.
Thévenod F, Wolff NA, Bork U, Lee WK, Abouhamed M. Cadmium induces nuclear translocation of beta-catenin and increases expression of c-myc and Abcb1a in kidney proximal tubule cells. Biometals. 2007;20:807–820.
Shaker OG, Moustafa W, Essmat S, Abdel-Halim M, El-Komy M. The role of interleukin-12 in the pathogenesis of psoriasis. Clin Biochem. 2006;39:119–125.
Chien AJ, Moon RT. WNTs and WNT receptors as therapeutic tools and targets in human disease processes. Front Biosci. 2007;12:448–457.
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538.
Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse disheveled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem. 1999;274:21464–21470.
Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated biquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738.
Behrens J, von Kries JP, Kühl M, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642.
Molenaar M, van de Wetering M, Oosterwegel M, et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399.
Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109.
Katoh M. Expression and regulation of WNT1 in human cancer: up-regulation of WNT1 by beta-estradiol in MCF-7 cells. Int J Oncol. 2003;22:209–212.
Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506.
Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480.
Goss KH, Groden J. Biology of the adenomatous polyposis coli tumor suppressor. J Clin Oncol. 2000;18:1967–1979.
Shih IM, Yu J, He TC, Vogelstein B, Kinzler KW. The β-catenin binding domain of adenomatous polyposis coli is sufficient for tumor suppression. Cancer Res. 2000;60:1671–1676.
Henderson BR. Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover. Nat Cell Biol. 2000;2:653–660.
Rosin-Arbesfeld R, Townsley F, Bienz M. The APC tumor suppressor has a nuclear export function. Nature. 2000;406:1009–1012.
Korinek V, Barker N, Morin PJ, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787.
Kikuchi A. Roles of Axin in the Wnt signalling pathway. Cell Signal. 1999;11:777–788.
Satoh S, Daigo Y, Furukawa Y, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus mediated transfer of AXIN1. Nat Genet. 2000;24:245–250.
He TC, Sparks A, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512.
Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426.
Zhao JJ, Gjoerup OV, Subramanian RR, et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495.
Maeda K, Chung YS, Kang SM, et al. Cyclin D1 overexpression and prognosis in colorectal adenocarcinoma. Oncology. 1998;55:145–151.
Said TK, Medina D. Cell cyclins and cyclin dependent kinase activities in mouse mammary tumor development. Carcinogenesis. 1995;16:823–830.
Hur K, Kim JR, Yoon BI, et al. Overexpression of cyclin D1 and cyclin E in 1,2-dimethylhydrazine dihydrochloride-induced rat colon carcinogenesis. J Vet Sci. 2000;1:121–126.
Collier JJ, Doan TT, Daniels MC, Schurr JR, Kolls JK, Scott DK. c-Myc is required for the glucose-mediated induction of metabolic enzyme genes. J Biol Chem. 2003;278:6588–6595.
Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: insights through mathematical models. Cancer Res. 2003;63:3847–3854.
Yamada N, Noguchi S, Mori T, Naoe T, Maruo K, Akao Y. Tumor-suppressive microRNA-145 targets catenin δ-1 to regulate Wnt/β-catenin signaling in human colon cancer cells. Cancer Lett. 2013 Mar 7. pii: S0304-3835(13)00223-1. doi:10.1016/j.canlet.2013.02.060.
Adams LS, Seeram NP, Aggarwal BB, Takada Y, Sand D, Heber D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J Agric Food Chem. 2006;54:980–985.
Sharma M, Li L, Celver J, Killian C, Kovoor A, Seeram NP. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin A, on Wnt signaling. J Agric Food Chem. 2010;58:3965–3969.
Ogata Y, Murakami H, Sasatomi T, et al. Elevated preoperative serum carcinoembrionic antigen level may be an effective indicator for needing adjuvant chemotherapy after potentially curative resection of stage II colon cancer. J Surg Oncol. 2009;99:65–70.
Sekiguchi Y, Nakaniwa T, Kinoshita T, et al. Structural insight into human CK2R in complex with the potent inhibitor ellagic acid. Bioorg Med Chem Lett. 2009;19:2920–2923.
Dominguez I, Sonenshein GE, Seldin DC. Protein kinase CK2 in health and disease: CK2 and its role in Wnt and NF-κB signaling: linking development and cancer. Cell Mol Life Sci. 2009;66:1850–1857.
Selma MV, Espı′n JC, Tomás-Barberán FA. Interaction between phenolics and gut microbiota: role in human health. J Agric Food Chem. 2009;57:6485–6501.
Seeram NP, Aronson WJ, Zhang Y, et al. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J Agric Food Chem. 2007;55:7732–7737.
Acknowledgments
The authors acknowledge Dr Osama Helmy, Pathology Department, National Cancer Institute, Cairo University, Cairo, Egypt for performing the histopathological examinations of this study. The present work was supported by the financial assistance provided by Faculty of Pharmacy, Cairo University, Cairo, Egypt.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sadik, N.A.H., Shaker, O.G. Inhibitory Effect of a Standardized Pomegranate Fruit Extract on Wnt Signalling in 1, 2-Dimethylhydrazine Induced Rat Colon Carcinogenesis. Dig Dis Sci 58, 2507–2517 (2013). https://doi.org/10.1007/s10620-013-2704-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2704-z