Abstract
Background and Aims
Daikenchuto, a traditional Japanese herbal medicine, suppresses bacterial translocation by improvement of gastrointestinal motility and blood flow. As Daikenchuto reportedly reduces gastrointestinal inflammatory activity by these mechanisms, we analyzed whether Daikenchuto suppresses experimental colitis and reduces inflammatory cytokine expression in a mouse model.
Methods
Colitis was induced by transfer of naive CD4+ T cells of BALB/c mice into SCID mice, and mice were given either control or 2.7 % Daikenchuto-containing feed. We investigated body weight, clinical symptoms, histological changes, and Th1- and Th17-cytokine expression. Cytokine mRNA expression was analyzed using real-time RT-PCR. The ratio of IL-17+ and IFN-γ+ CD4+ T cells were analyzed by flow cytometry.
Results
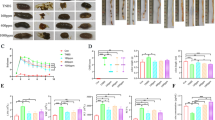
Daikenchuto delayed the development of colitis and significantly reduced the histological inflammation scores. Analyses of cytokine mRNA revealed that Th17 cytokines were significantly decreased in colons of mice that received Daikenchuto. Absolute numbers of IL-17+ or IFN-γ+ CD4+ T cells per colon were less in mice receiving Daikenchuto than in mice that received control feed, as both groups received naive CD4+ T cells to induce colitis.
Conclusions
We demonstrated that feeding administration of Daikenchuto suppresses colitis induced by naive CD4+ T cell transfer into SCID mice. Daikenchuto may show clinical benefit in the treatment of human inflammatory bowel disease and further studies are warranted.




Similar content being viewed by others
References
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205.
Lakatos P. Environmental factors affecting inflammatory bowel diseases: have we made progress? Dig Dis. 2009;27:215–225.
Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434.
Ardizzone S, Cassinotti A, Manes G, et al. Immunomodulators for all patients with inflammatory bowel disease? Therap Adv Gastroenterol. 2010;3:31–42.
Ito T, Yamakawa J, Mai M, et al. The effect of the herbal medicine dai-kenchu-to on post-operative ileus. J Int Med Res. 2002;30:428–432.
Tokita Y, Yuzurihara M, Sakaguchi M, et al. The pharmacological effects of Daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in Rat postoperative ileus. J Pharmacol Sci. 2007;104:303–310.
Sato K, Kase Y, Yuzurihara M, et al. Effect of Dai-kenchu-to (Da-Jian-Zhong-Tang) on the delayed intestinal propulsion induced by chlorpromazine in mice. J Ethnopharmacol. 2003;86:37–44.
Murata P, Kase Y, Ishige A, et al. The herbal medicine Dai-kenchu-to and one of its active components 6-shogaol increase intestinal blood flow in rats. Life Sci. 2002;70:2061–2070.
Takayama S, Seki T, Watanabe M, et al. The herbal medicine daikenchuto increases blood flow in the superior mesenteric artery. Tohoku J Exp Med. 2009;219:319–330.
Quigley EM. Microflora modulation of motility. J Neurogastroenterol Motil. 2011;17:140–147.
Yoshikawa K, Kurita N, Higashijima J, et al. Kampo medicine “Dai-Kenchu-To” prevents bacterial translocation in rats. Dig Dis Sci. 2008;53:1824–1831.
Kono T, Kaneko A, Hira Y, et al. Anti-colitis and -adhesion effects of daikenchuto via endogenous adrenomedullin enhancement in Crohn’s disease mouse model. J Crohns Colitis. 2010;4:161–170.
Ogino H, Nakamura K, Ihara E, et al. CD4(+)CD25(+) regulatory T cells suppress Th17-responses in an experimental colitis model. Dig Dis Sci. 2011;56:376–386.
Mudter J, Wirtz S, Galle PR, Neurath MF. A new model of chronic colitis in SCID mice induced by adoptive transfer of CD62L + CD4 + T cells: insights into the regulatory role of interleukin-6 on apoptosis. Pathobiology. 2002;70:170–176.
Fantini MC, Becker C, Tubbe I, et al. Transforming growth factor β induced FoxP3 + regulatory T cells suppress Th1 mediated experimental colitis. Gut. 2006;55:671–680.
Siegmund B, Rieder F, Aldrich S, et al. Adenosine kinase inhibitor GP515 improves experimental colitis in mice. J Pharmacol Exp Ther. 2001;296:99–105.
Honda K, Nakamura K, Matsui N, et al. T helper 1-inducing property of IL-27/WSX-1 signaling is required for the induction of experimental colitis. Inflamm Bowel Dis. 2005;11:1044–1052.
Yu Y, Sitaraman S, Gewirtz AT. Intestinal epithelial cell regulation of mucosal inflammation. Immunol Res. 2004;29:5–68.
Berg RD. Bacterial translocation from the gastrointestinal tract. J Med. 1992;23:217–244.
Alexander JW, Boyce ST, Babcock GF, et al. The process of microbial translocation. Ann Surg. 1990;212:496–512.
MacFie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006;93:87–93.
Wang YB, Liu J, Yang ZX. Effects of intestinal mucosal blood flow and motility on intestinal mucosa. World J Gastroenterol. 2011;17:657–661.
Shultz M, Strauch UG, Linde HJ, et al. Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol. 2004;11:372–378.
Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625.
Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel disease: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633.
Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623.
Infante-Duarte C, Horton HF, Byrne MC, et al. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–6115.
Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573.
Nakae S, Nambu A, Sudo K, et al. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177.
Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316.
Elson CO, Conq Y, Weaver CT, et al. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370.
Kobayashi T, Okamoto S, Hisamatsu T, et al. IL-23 differentially the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682–1689.
Hölttä V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175–1184.
Acknowledgments
We thank Mr. Yohei Tokita and Dr. Masahiro Yamamoto for technical assistance. This work was supported in part by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwasa, T., Ogino, H., Nakamura, K. et al. Feeding Administration of Daikenchuto Suppresses Colitis Induced by Naive CD4+ T Cell Transfer into SCID Mice. Dig Dis Sci 57, 2571–2579 (2012). https://doi.org/10.1007/s10620-012-2218-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2218-0