Abstract
Background
Infliximab-induced hepatotoxicity is reported in several case studies involving patients with inflammatory bowel disease (IBD) and a direct hepatotoxic effect has been proposed.
Objective
The aim of this study was to determine the direct in vitro toxicity of infliximab. As a proof of principle the in vitro toxicity of thiopurines and methotrexate was also determined.
Methods
Cell survival curves and the half maximal inhibitory concentrations (IC50) were obtained after 24, 48 and 72 h of incubation in HepG2 cells with the IBD drugs azathioprine, 6-mercaptopurine, 6-thioguanine, methotrexate or infliximab by using the WST-1 cytotoxicity assay.
Results
No in vitro hepatotoxicity in HepG2 cells was seen with infliximab, while concentration-dependent cytotoxicity was observed when HepG2 cells were incubated with increasing concentrations of azathioprine, 6-mercaptopurine and 6-thioguanine.
Conclusion
Infliximab alone or given in combination with azathioprine showed no direct hepatotoxic effect in vitro, indicating that the postulated direct hepatotoxicity of infliximab is unlikely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
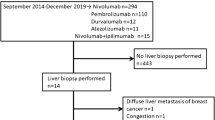
Hepatotoxicity is defined as injury to the liver that is associated with impaired liver function caused by exposure to a drug or another noninfectious agent [1]. It is a serious complication, frequently observed in the medical treatment of inflammatory bowel disease (IBD) [2]. It can be directly attributed to the type of drugs used to treat IBD, such as immunosuppressants or biological therapies targeting TNF-α. However, hepatotoxicity may also result from drugs used to treat complications of immunosuppressants and TNF-α antagonists, e.g. isoniazid for the treatment of reactivation tuberculosis, or may be a result of exacerbation of underlying chronic viral hepatitis caused by immunosuppression [3].
In December 2004, the Food and Drug Administration (FDA) issued a drug warning to alert health care professionals to the risk of hepatotoxicity linked to infliximab [4]. However, severe hepatic reactions, including acute liver failure, jaundice, hepatitis or cholestasis have rarely been reported in patients receiving infliximab [5–8]. Furthermore, as demonstrated by Sokolove et al. [6] elevations of serum transamines in patients receiving biological therapy are uncommon and abnormalities of more than two times the upper limit of normal are rarely observed. The mechanism of infliximab-induced hepatotoxicity is poorly understood, although a direct hepatoxic effect has been proposed by several authors [6–8]. To our knowledge, no in vivo or in vitro results supporting this hypothesis have been reported.
The aim of this study was to determine the in vitro hepatotoxicity of infliximab. As a proof of principle, the conventional IBD medication, i.e. the thiopurines azathioprine, 6-mercaptopurine and 6-thioguanine and methotrexate, which are all known to be hepatotoxic, were also tested. Although cultures of primary human hepatocytes seem to have the most relevant physiological properties for the evaluation of in vitro IBD drug hepatotoxicity, they are difficult to obtain and rapidly lose their metabolic properties [9]. Therefore, we used a human liver hepatocellular carcinoma (HepG2) cell line, which is very stable, easy to handle and previously used in drug toxicity studies [9]. HepG2 cells were incubated with increasing concentrations of infliximab, methotrexate or thiopurines for 24, 48 or 72 h and subsequently cell viability was determined.
Materials and Methods
Cell Culture
Human hepatocellular carcinoma (HepG2) cells (American Type Culture Collection, Rockville, Maryland, USA) were grown in Dulbecco’s Modified Eagle Medium (DMEM, PAA Laboratories GmbH, Pasching, Austria) containing 10 % (v/v) heat-inactivated fetal bovine serum (Gibco Invitrogen, Paisley, Scotland), 1X non-essential amino acids (PAA), 20 mM HEPES buffer (PAA) and 50 mg/l gentamycin (Gibco) at 37 °C in a humidified atmosphere containing 5 % CO2. The medium was renewed every 3 days and when confluence was reached, cells were harvested with trypsin/EDTA (Cambrex, Verviers, Belgium), washed with phosphate-buffered saline, and used for cytotoxicity assays.
Cytotoxicity Assays
HepG2 cells were seeded in flat-bottomed 96-well microtitre plates (Costar; Corning Inc., Corning, New York, USA) at a density of 5.0 × 104 cells per well in a final volume of 100 μl culture medium, and cells were cultured for 24 h. Subsequently, cells were incubated with the single drugs azathioprine, 6-mercaptopurine, methotrexate (all Sigma-Aldrich Chemie B.V., Zwijndrecht, The Netherlands), 6-thioguanine (Alfa Aesar GmbH & Co KG, Karlsruhe, Germany) or infliximab (Remicade©, Centocor, Leiden, the Netherlands) for 24, 48 and 72 h. The following concentration series were used: azathioprine: 0.002 μM–4 mM; 6-mercaptopurine: 0.002–200 μM; 6-thioguanine: 0.002 μM–4 mM; methotrexate: 50 nM–100 μM; infliximab: 0.002 mg/l–5 g/l.
In subsequent experiments the cytotoxicity of a combination of drugs was tested. A single, non-toxic concentration of azathioprine (1 μM) was tested in combination with a concentration range of infliximab (0.002 mg/l–5 g/l), whereas a single, non-toxic concentration of infliximab (312 mg/l) was tested in combination with a concentration range of azathioprine (0.002 μM–4 mM).
All drugs were first dissolved in 0.1 M NaOH and then rapidly diluted in the culture medium to reach the final concentration. A refreshment of the culture medium with the various concentrations of drugs was done every 24 h. After the incubation with drugs, a cell survival assessment was performed by adding water-soluble tetrazolium salt-1 (WST-1, Roche Diagnostics Nederland BV, Almere, The Netherlands) according to the manufacturer’s instructions. In the WST-1 assay, tetrazolium salts are cleaved by dehydrogenases of viable cells to produce formazan. The amount of formazan dye was quantified by using an ELISA plate reader at 440 nm. Cell survival was defined as:
where A experimental is the absorbance of drug incubated cells plus WST-1, A background is the absorbance of culture medium plus WST-1 in the absence of cells and A control is the absorbance of cells without drugs plus WST-1. Test results were obtained from three independent experiments, each performed in triplicate.
Statistics
One-way ANOVA analysis was used to compare the effect of incubation time. A p-value <0.05 was considered statistically significant. Data were normalized to untreated cells using GraphPad Prism to calculate the concentration at which cell survival is 50 % (IC50, i.e. half maximal inhibitory concentrations). All statistical analyses and calculations were carried out using GraphPad Prism (version 4.0; GraphPad Software, San Diego, California, USA).
Results
The incubation of HepG2 cells with increasing concentrations of infliximab did not result in significant changes in cell viability, indicating that a direct in vitro cytotoxic effect was absent (Fig. 1). At all concentrations of infliximab tested after 24, 48 or 72 h of incubation, cell survival was above 50 % and no IC50 values could be determined (Table 1). Only at the highest concentration of 5 g/l, could a decrease of about 30 % in cell survival could be seen (Fig. 1). Concentration-dependent cytotoxicity was clearly observed when HepG2 cells were incubated with increasing concentrations of azathioprine, 6-mercaptopurine or 6-thioguanine (Fig. 2). Only methotrexate showed a time-dependent cytotoxic effect on HepG2 cells. Incubation with various concentrations of methotrexate for 72 h resulted in a significant difference when compared to 24 h of incubation (p < 0.01).
Effect of thiopurines and methotrexate on HepG2 cell viability. HepG2 cells were incubated with various concentrations of IBD drugs for 24, 48 or 72 h and cell survival was measured and expressed as percentage of untreated cells. The graphs summarize the results of three independent experiments (means ± SEM), performed in triplicate
After 24 h of incubation, neither the combination of infliximab (0.002 mg/l–5 g/l) with a low dose (1 μM) of azathioprine nor the combination of a single, non-toxic concentration of infliximab (312 mg/l) in combination with azathioprine (0.002 μM–4 mM) showed any difference in cell survival (data not shown).
Discussion
In this study infliximab showed no direct cytotoxic effect on HepG2 cells, even at concentrations far exceeding the maximum concentration of 118 μg/ml, which infliximab achieves when administered intravenously at a dosage of 5 mg/kg [10]. Concomitant incubation with both infliximab in different dosages and azathioprine at a non toxic concentration did not alter HepG2 cell viability. Our in vitro results therefore suggest that a direct hepatotoxicity of infliximab is implausible. Alternatively, infliximab-induced hepatotoxicity is more likely to be immuno-mediated or induced via Fc receptor-mediated interactions. After forming an immune complex with TNF-α, this complex is cleared by the mononuclear phagocytic system in the liver via Fc receptor-mediated interactions that in turn can activate Kupffer cells. These resident macrophages of the liver located in hepatic sinusoids do release reactive oxygen species which may lead to local damage of hepatocytes [11–13]. During infliximab therapy, increased formation of anti-nuclear antibodies has been observed [14], most possibly due to the fact that binding of infliximab to transmembrane TNF on the cell surface induces apoptosis, leading to the release of nucleosomes and the generation of anti-nuclear antibodies [15]. Since antibodies to TNF-α delay the repair of liver injury [16, 17], the use of infliximab might also exacerbate a previous suboptimal liver condition not recognized by any clinical symptoms or biochemical markers. Furthermore, a potential hepato-protective effect of TNF-α induced by increasing hepatocyte regeneration and decreasing apoptosis has been observed in a transgenic mouse model of chronic hepatitis C while treatment with anti-TNF-α blocked the anti-apoptotic and regenerative effects induced by TNF-α [18].
In contrast to our experience with infliximab, we observed a concentration dependent cytotoxic effect of the thiopurines in HepG2 cells, while methotrexate demonstrated a time- and concentration-dependent effect. The in vitro hepatotoxic effects of thiopurines have also been demonstrated by Petit et al., comparing the cytotoxicity of thiopurines in human hepatocytes and HepaRG cells, incubated for 24, 48, 72 and 96 h with 1, 5 or 25 μM of azathioprine, 6-mercaptopurine or 6-thioguanine. They reported a dose- and time-dependent cytotoxic effect of azathioprine and 6-mercaptopurine in both human hepatocytes and HepRG cells, while 6-thioguanine had no significant effect on human hepatocytes. However, 72 h of incubation with either 5 or 25 μM of 6-thioguanine showed a 30 % decrease in cell survival of HepaRG cells [19].
The observed time-dependent cytotoxic effect of methotrexate in our study is in line with results of Yin et al. [20] who reported a time- and concentration-dependent effect of high dose methotrexate (1–10 mM) in rat hepatocytes. These concentrations however go far beyond the mean peak concentration in human plasma of 1.14 μM achieved after subcutaneous administration of 15 mg methotrexate to patients with IBD [21].
Several limitations of our study should be noticed. First of all, results of in vitro studies cannot be directly extrapolated to the in vivo situation. Isolated liver (carcinoma) cells will respond differently to stress or toxic compounds than to an intact and perfused liver. Therefore, although results from cell lines add to the understanding of drug-induced toxicity, they will be difficult to translate into clinical practice. Processes of absorption, distribution, metabolism and excretion, which determine the exposure of the target tissues of an organism in vivo, are mainly absent in in vitro studies [22]. Furthermore, peripheral serum or plasma concentrations do not reflect the concentrations in the portal vein. Therefore the drug concentrations to which the liver is actually exposed are largely unknown. These points are especially true for the thiopurines. Resorption is highly variable in animal studies as well as in patients with inflammatory bowel disease and the pro-drugs azathioprine and 6-mercaptopurine are rapidly converted [23–26]. Additionally, as in hepatocytes in the primary culture, changes in the expression of drug metabolizing enzymes over time occur in HepG2 cells [27]. Therefore neither primary cultures of hepatocytes nor HepG2 cells display an ideal model mimicking the expression levels of drug metabolizing enzymes as present in hepatocytes in vivo, thereby limiting the reproducibility of in vitro hepatotoxicity experiments using different cell cultures [27]. In our study we focused on cell viability as a marker of hepatotoxicity. Alterations in pathways underlying cell death including oxidative stress were not studied.
In conclusion, our study suggests that infliximab does not have a direct toxic effect on HepG2 cells. In addition, infliximab in combination with thiopurines does not increase their in vitro toxicity on HepG2 cells. Our results may not be translated to clinical practice directly without considering the limitations of these findings. On the other hand, no alarming cytotoxicity is seen in the same assay that shows evident dose-related thiopurine cytotoxicity. Future studies regarding the hepatotoxic effects of infliximab should focus on Fc receptor-mediated interactions and auto-immune related factors.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- CD:
-
Crohn’s disease
- UC:
-
Ulcerative colitis
- TNF-α:
-
Tumour necrosis factor alpha
References
Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739.
Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157–165.
Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28:508–518.
US Food and Drug Association (2004) MedWatch safety alerts for human medical products. http://www.fda.gov/medWatch/safety/2004/remicade_DHCP_dec04.pdf. Accessed 5 April 2012
Federal Drug Administration (2009) REMICADE® (infliximab) for IV Injection. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/103772s5234lbl.pdf. Accessed 5 April 2012
Sokolove J, Strand V, Greenberg JD, et al. Risk of elevated liver enzymes associated with TNF inhibitor utilisation in patients with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1612–1617.
Mancini S, Amorotti E, Vecchio S, et al. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern Emerg Med. 2010;5:193–200.
Ierardi E, Valle ND, Nacchiero MC, et al. Onset of liver damage after a single administration of infliximab in a patient with refractory ulcerative colitis. Clin Drug Investig. 2006;26:673–676.
Yeon JH, Na D, Park JK. Hepatotoxicity assay using human hepatocytes trapped in microholes of a microfluidic device. Electrophoresis. 2010;31:3167–3174.
Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–279.
Johansson AG, Sundqvist T, Skogh T. IgG immune complex binding to and activation of liver cells. An in vitro study with IgG immune complexes, Kupffer cells, sinusoidal endothelial cells and hepatocytes. Int Arch Allergy Immunol. 2000;121:329–336.
Aithal GP. Hepatotoxicity related to antirheumatic drugs. Nat Rev Rheumatol. 2011;7:139–150.
Rojas JR, Taylor RP, Cunningham MR, et al. Formation, distribution, and elimination of infliximab and anti-infliximab immune complexes in cynomolgus monkeys. J Pharmacol Exp Ther. 2005;313:578–585.
Ferraro-Peyret C, Coury F, Tebib JG, et al. Infliximab therapy in rheumatoid arthritis and ankylosing spondylitis-induced specific antinuclear and antiphospholipid autoantibodies without autoimmune clinical manifestations: a two-year prospective study. Arthritis Res Ther. 2004;6:R535–R543.
D’Auria F, Rovere-Querini P, Giazzon M, et al. Accumulation of plasma nucleosomes upon treatment with anti-tumour necrosis factor-alpha antibodies. J Intern Med. 2004;255:409–418.
Akerman P, Cote P, Yang SO, et al. Antibodies to tumor necrosis factor-alpha inhibit liver regeneration after partial hepatectomy. Am J Physiol. 1992;263:G579–G585.
Bruccoleri A, Gallucci R, Germolec DR, et al. Induction of early-immediate genes by tumor necrosis factor alpha contribute to liver repair following chemical-induced hepatotoxicity. Hepatology. 1997;25:133–141.
Brenndörfer ED, Weiland M, Frelin L, et al. Anti-tumor necrosis factor α treatment promotes apoptosis and prevents liver regeneration in a transgenic mouse model of chronic hepatitis C. Hepatology. 2010;52:1553–1563.
Petit E, Langouet S, Akhdar H, et al. Differential toxic effects of azathioprine, 6-mercaptopurine and 6-thioguanine on human hepatocytes. Toxicol In Vitro. 2008;22:632–642.
Yin J, Meng Q, Zhang G, et al. Differential methotrexate hepatotoxicity on rat hepatocytes in 2-D monolayer culture and 3-D gel entrapment culture. Chem Biol Interact. 2009;180:368–375.
Egan LJ, Sandborn WJ, Mays DC, et al. Systemic and intestinal pharmacokinetics of methotrexate in patients with inflammatory bowel disease. Clin Pharmacol Ther. 1999;65:29–39.
Tostmann A, Boeree MJ, Peters WHM, et al. Isoniazid and its toxic metabolite hydrazine induce in vitro pyrazinamide toxicity. Int J Antimicrob Agents. 2008;31:577–580.
Zimm S, Collins JM, Riccardi R, et al. Variable bioavailability of oral mercaptopurine: is maintenance chemotherapy in acute lymphoblastic leukemia being optimally delivered? N Engl J Med. 1983;308:1005–1009.
Ding TL, Benet LZ. Comparative bioavailability and pharmacokinetic study of azathioprine and 6-mercaptopurine in the rhesus monkey. Drug Metab Dispos. 1979;7:373–377.
Deibert P, Dilger K, Fischer C, et al. High variation of tioguanine absorption in patients with chronic active Crohn’s disease. Aliment Pharmacol Ther. 2003;18:183–189.
Chan GL, Canafax DM, Johnson CA. The therapeutic use of azathioprine in renal transplantation. Pharmacotherapy. 1987;7:165–177.
Wilkening S, Bader A. Influence of culture time on the expression of drug-metabolizing enzymes in primary human hepatocytes and hepatoma cell line HepG2. J Biochem Mol Toxicol. 2003;17:207–213.
Conflicts of interest
Dirk J. de Jong has received fees for speaking, organizing education, consultancy and research from Abbott Nederland, Schering Plough, Falk Pharma GmbH, UCB Pharma, Ferring BV, Nycomed, Synthon Nijmegen, Vifor Pharma, Tramedico Weesp and Shire pharmaceuticals. All other authors declare no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Vries, H.S., de Heij, T., Roelofs, H.M.J. et al. Infliximab Exerts No Direct Hepatotoxic Effect on HepG2 Cells In Vitro. Dig Dis Sci 57, 1604–1608 (2012). https://doi.org/10.1007/s10620-012-2159-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2159-7