Abstract
Background
Surveillance of premalignant gastric lesions relies mainly on random biopsy sampling. Narrow band imaging (NBI) may enhance the accuracy of endoscopic surveillance of intestinal metaplasia (IM) and dysplasia. We aimed to compare the yield of NBI to white light endoscopy (WLE) in the surveillance of patients with IM and dysplasia.
Methods
Patients with previously identified gastric IM or dysplasia underwent a surveillance endoscopy. Both WLE and NBI were performed in all patients during a single procedure. The sensitivity of WLE and NBI for the detection of premalignant lesions was calculated by correlating endoscopic findings to histological diagnosis.
Results
Forty-three patients (28 males and 15 females, mean age 59 years) were included. IM was diagnosed in 27 patients; 20 were detected by NBI and WLE, four solely by NBI and three by random biopsies only. Dysplasia was detected in seven patients by WLE and NBI and in two patients by random biopsies only. Sixty-eight endoscopically detected lesions contained IM: 47 were detected by WLE and NBI, 21 by NBI only. Nine endoscopically detected lesions demonstrated dysplasia: eight were detected by WLE and NBI, one was detected by NBI only. The sensitivity, specificity, positive and negative predictive values for detection of premalignant lesions were 71, 58, 65 and 65% for NBI and 51, 67, 62 and 55% for WLE, respectively.
Conclusions
NBI increases the diagnostic yield for detection of advanced premalignant gastric lesions compared to routine WLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Helicobacter pylori is an important risk factor for gastric cancer due to the fact that it causes chronic inflammation of the gastric mucosa in virtually all infected patients. In the multi-step model of gastric carcinogenesis, this chronic inflammation may slowly progress through the pre-malignant stages of atrophic gastritis, intestinal metaplasia and dysplasia to gastric adenocarcinoma [1]. We have previously shown that the actual cancer risk for patients with any of the pre-malignant conditions of the stomach is very similar to the cancer risk in patients with a Barrett’s oesophagus or in those after removal of colonic adenoma [2]. Surveillance of these pre-malignant lesions could lead to early detection of patients with disease progression, and thus to early intervention aiming at cancer prevention and improved survival of these patients. However, recent investigation has demonstrated that current surveillance of pre-malignant gastric lesions is at great discrepancy with the substantial gastric cancer risk of these lesions [2]. Furthermore, studies have shown that a considerable proportion of patients with dysplastic lesions are being missed in current routine gastroenterology practice [3].
The gold standard for diagnosing these gastric lesions is histology of biopsy specimens. The major shortcoming of this approach is the fact that pre-malignant lesions may occur multifocally, and may thus be missed on random biopsy sampling. Although image quality of standard endoscopes has improved dramatically over the past decades, endoscopic evaluation of the condition of the gastric mucosa still correlates poorly with histological findings [4–7]. Therefore, a diagnosis of pre-malignant gastric lesions remains dependent on random biopsy sampling during conventional gastroscopy.
Several new imaging techniques to overcome limitations of conventional white light endoscopy (WLE) have been developed over recent decades. A promising technique is narrow-band imaging (NBI). The principle of this new technique is based on modification of the spectral characteristics of the optical filter in the light source, which leads to improved visibility of mucosal structures. With the use of different narrow-band filters in combination with image magnification, mucosal structures can be very clearly demonstrated, among others, resulting in increased contrast between surface and vascular pattern [8].
Several studies described the diagnostic accuracy of NBI in detecting gastrointestinal lesions [8, 9]. Based on these results, one might expect that the use of this technique for targeted biopsy sampling can increase the diagnostic yield of endoscopy for primary detection of pre-malignant gastric lesions. However, the additional value of NBI in the surveillance of patients with pre-malignant gastric lesions is yet unclear and requires further investigation.
Therefore the aim of this study was to compare the yield of NBI over conventional white light endoscopy (WLE) in the surveillance of patients with intestinal metaplasia and dysplasia, using histology as a reference value.
Methods
This single center, prospective study was carried out in the Erasmus MC in Rotterdam. The study protocol was approved by the local Institutional Review Board. All patients provided informed consent.
Patient Selection and Endoscopic Procedure
Patients with previously identified intestinal metaplasia or dysplasia of the gastric mucosa underwent a surveillance endoscopy. Patients with coagulopathy uncorrected at the time of endoscopy or a thrombocytopenia (<50 × 109/l) were excluded. After informed consent, both WLE and NBI were performed in all patients by a single endoscopist specialized in NBI and WLE endoscopy of the gastric mucosa during a single procedure with a GIF180 endoscope (Olympus Optical, Hamburg, Germany). The procedure started with conventional white light endoscopy. During endoscopy, all suspicious antral and angular gastric lesions were photographed, videotaped, and documented on a specially designed scoring sheet in terms of size (cm) and morphology (according to the Paris classification). During the same setting, the stomach was carefully observed using the NBI system. Again, all suspicious antral and angular gastric lesions were photographed, videotaped, and documented on the specially designed scoring sheet. NBI suspicious lesions for intestinal metaplasia were defined as bluish-whitish areas with an irregular mucosal pattern, a complete loss of architectural and mucosal pattern was suspicious for dysplasia. At least one targeted biopsy was taken from all endoscopic lesions suspected for intestinal metaplasia or dysplasia by NBI or WLE. Furthermore, four random biopsies were obtained: two from the antrum and two from the angulus.
Histological Assessment
Biopsy specimens obtained from the stomach were fixed in buffered formalin and embedded in paraffin. The specimens were sectioned at 4-μm thickness, and stained by haematoxylin and eosin. An expert pathologist specialized in GI pathology reviewed the sections and was blinded to endoscopic and clinical findings. Inflammation, atrophy, metaplasia and dysplasia were classified according to the updated Sydney System and revised Vienna classification [10–12].
Statistical Analysis
The number of patients with intestinal metaplasia or dysplasia detected by NBI and WLE was evaluated. Furthermore, the number of endoscopically detected lesions which were suspected for intestinal metaplasia or dysplasia by NBI and/or WLE were evaluated. These endoscopically suspected lesions were considered as the unit of analysis in this evaluation, even though some patients had more than one lesion. For the random biopsies, the overall diagnosis of the antrum biopsies and the overall diagnosis of the angulus biopsies were considered as unsuspected lesions by NBI or WLE. Each endoscopically suspected lesion (identified by NBI and WLE) or unsuspected lesion (random biopsy) was considered as an independent observation for statistical purposes. For each patient and each lesion only the most severe pre-malignant grading was evaluated. For instance, patients with intestinal metaplasia and with a concomitant diagnosis of dysplasia were classified as having dysplasia.
Sensitivity, specificity, positive and negative predictive values for the prediction of intestinal metaplasia and dysplasia for NBI and WLE were calculated using histology as a reference value. Differences between NBI and WLE were assessed by using McNemar’s test and by analyzing receiver operating characteristic (ROC) curves. In addition, a bootstrap resampling model (using c-stat) was performed to calculate the difference between the discriminatory power of both techniques [13, 14]. The data were submitted for statistical testing using the Statistical Package for the Social Sciences (SPSS), version 11.0.
Results
From May 2007 until December 2008, 43 patients (28 males, 15 females) with a mean age of 58.7 years (range 34–75 years) were included. Of these patients, 32 (74%) patients had a previous diagnosis of intestinal metaplasia and 11 (26%) patients had a previous diagnosis of dysplasia. The majority of patients (88%) were of Dutch descent. The baseline characteristics of the patients are presented in Table 1. The mean interval between initial diagnosis and surveillance endoscopy was 2.0 years (range 0.8–21.1 years) for patients with intestinal metaplasia and 1.9 years (range 0.2–5.2 years) for patients with dysplasia.
Per Patient Analysis
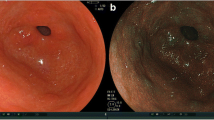
Of the 43 patients that were included, 27 (63%) demonstrated intestinal metaplasia at surveillance endoscopy and nine (21%) patients demonstrated dysplasia (low grade dysplasia n = 6; high grade dyplasia n = 3) (Table 2; Figs. 1 and 2). In the remaining seven (16%) patients no diagnosis of intestinal metaplasia or dysplasia was confirmed at surveillance endoscopy. Baseline endoscopy had shown intestinal metaplasia in antrum and corpus mucosa in five (12%) and two (4%) of these patients, respectively.
Of the 27 intestinal metaplasia patients, 20 (74%) were detected by both WLE and NBI, whereas four (15%) were detected solely by NBI. The remaining three (11%) patients were detected by random biopsy sampling only. Of the nine dysplasia patients, seven (78%) patients (low grade dysplasia n = 4 and high grade dysplasia n = 3) were detected by both WLE and NBI and not by random biopsies. In the remaining two (22%) patients, random biopsies demonstrated foci with low grade dysplasia; NBI and WLE detected marked intestinal metaplasia but no dysplasia in these two patients.
Per Lesion Analysis
In total, 121 lesions in the gastric mucosa were endoscopically suspected for intestinal metaplasia or dysplasia after WLE and NBI (Table 3). Eighty-six (71%) of these endoscopic lesions were suspected for intestinal metaplasia or dysplasia by both WLE and NBI. Two (2%) were only suspected by WLE, and 33 (27%) were only suspected by NBI.
Seventy-seven (64%) of these 121 endoscopically suspected lesions had a histopathological diagnosis of intestinal metaplasia (n = 68) or dysplasia (n = 9). For intestinal metaplasia (n = 68), 47 (69%) endoscopical lesions were detected both by WLE and NBI, the remaining 21 (30%) lesions were solely detected by NBI. For dysplasia (n = 9), eight (89%) endoscopic lesions (low grade dysplasia n = 5, and high grade dysplasia n = 3) were detected by WLE and NBI, whereas one (11%) lesion with low grade dysplasia was detected by NBI only.
Although all 121 endoscopic lesions were suspected for intestinal metaplasia or dysplasia by NBI or WLE, 44 (36%) of these 121 suspected lesions did not show intestinal metaplasia or dysplasia when histologically assessed. Thirty-one (70%) of these false positive lesions were suspected both by WLE and NBI, two (5%) were suspected only by WLE, and 11 (25%) were suspected by NBI only.
The overall diagnosis of the random biopsies resulted in 27 additional endoscopically suspected lesions which showed intestinal metaplasia and 4 lesions which showed low grade dysplasia. All these regions had not been suspected endoscopically by NBI or WLE.
Based on these results, the sensitivity, specificity, positive and negative predictive values of WLE endoscopy were 51, 67, 62 and 55%, respectively. For NBI, the sensitivity, specificity, positive predictive value and negative predictive value were 71, 58, 65 and 65%, respectively. Specificity was marginally higher for WLE (P = 0.04), whereas sensitivity was considerably lower for WLE than for NBI (P < 0.001). In addition, according to bootstrap resampling, NBI was superior in detecting intestinal metaplasia and dysplasia versus normal mucosa than WLE (P = 0.03).
Discussion
This study provides evidence that NBI yields more accurate results in the surveillance of patients with intestinal metaplasia and dysplasia than conventional WLE. First, we demonstrated that 15% of the patients with intestinal metaplasia at surveillance endoscopy were solely detected by NBI. Second, considerably more endoscopically detected lesions with intestinal metaplasia were detected by NBI compared to WLE. And third, the sensitivity for the detection of advanced precursor lesions increased by 20–71% for NBI.
Similar to our observations, previous studies demonstrated promising results for NBI for the detection of pre-neoplastic lesions in the gastrointestinal tract, in particular for colon and esophagus [15]. The additional value of NBI in the detection of gastric pre-malignant lesions remained less clear, especially in countries with a low gastric cancer incidence.
A Japanese study described a sensitivity and specificity of 89 and 93%, respectively, for the detection of gastric intestinal metaplasia with NBI endoscopy [8]. This high accuracy compared to our findings is probably explained by training differences that exist between Japanese and Western gastroenterologists. Due to the high gastric cancer incidence, Japanese endoscopists are trained to scrutinize gastric mucosal areas which are compatible with atrophy and early cancer. Moreover, considerably more time is spent on a thorough mucosal examination, than in Western countries [16]. Another possible explanation for the high sensitivity and specificity found in the previous study was the use of NBI in combination magnification endoscopy [8]. In Japan, it has been demonstrated that magnification endoscopy can accurately detect gastric cancer during routine endoscopy [17–19]. However, our study shows that in Western countries with an overall low gastric cancer incidence, even without adding magnification, NBI endoscopy is of additional value for the detection of pre-malignant gastric lesions, in particular in a surveillance setting.
Currently, the diagnosis of intestinal metaplasia and dysplasia is based on histological evaluation of biopsy specimens. Since endoscopic diagnosis of pre-malignant lesions shows high interobserver variability and has poor correlation to histological diagnosis, numerous other endoscopic techniques have been developed to overcome these limitations in the last decades.
Similar to NBI, the use of auto-fluorescence endoscopy demonstrated a high correlation between Barrett’s esophagus and histological diagnosis. However, the correlation between gastric cancer and this imaging technique still remains controversial [20–22]. For chromoendoscopy, a previous study demonstrated a facilitated detection of early gastric cancer in hereditary diffuse gastric cancer [23]. Moreover, compared to auto-fluorescence endoscopy, the equipment necessary for chromoendoscopy is widely available and the technique is often quick and inexpensive. For some new staining techniques however, safety remains questionable [24].
Confocal endomicroscopy is a newly developed endoscopic technique that produces 1000-fold magnification cross-sectional images. This new technique can accurately predict the presence of early cancer in targeted areas, and a recently published gastric pit-pattern classification for the prediction for gastritis and atrophy showed a high correlation with histology [25, 26]. Nevertheless, confocal endomicroscopy is not able to completely replace histology and interobserver and intraobserver agreement for this pit-pattern classification remains unknown. Furthermore, the technique is too elaborate to be used for assessment of the complete gastric mucosa.
Compared to these new techniques, magnification endoscopy demonstrated the best sensitivities and specificities for a diagnosis of atrophic gastritis or gastric cancer. However, similar to NBI, most of these previous studies were of Japanese origin and mostly included low numbers of patients [17, 18, 27, 28]. In addition, despite the promising results of magnification endoscopy, uniform classification criteria of this technique have to date not been confirmed in large controlled trials in Western or Eastern countries.
Since our study shows that NBI has a low specificity and suboptimal sensitivity for the detection of preneoplastic gastric lesions, a combination of both NBI as well as magnification is likely to provide the best alternative with current endoscopical practice. Previous studies already demonstrated a high correlation between microvascular patterns found with NBI in combination with magnification, and a diagnosis of gastric cancer [9, 29]. Therefore, further research in a prospective study design is necessary to evaluate whether NBI in combination with magnification also yields adequate results for the detection and surveillance of patients with intestinal metaplasia or dysplasia in Western countries.
Previous studies demonstrated that surveillance of patients with pre-malignant gastric lesions should preferably be limited to patients at high risk of gastric cancer [2]. A risk score based on histology only (OLGIM staging system) or a broader risk classification including several clinical and laboratory parameters have been described [30, 31]. For either method, adequate biopsy sampling at baseline is essential. In this study we show that NBI has the potential to increase and optimize the yield of biopsies with intestinal metaplasia. However, although NBI shows an improved sensitivity for the detection of premalignant gastric lesions over WLE, random biopsy sampling is still necessary in the surveillance of patients with pre-malignant gastric lesions. This is illustrated by the fact that three patients with intestinal metaplasia and two patients with dysplasia were not detected after WLE and NBI endoscopy and were only diagnosed with these pre-malignant lesions after histological evaluation of the random biopsies. Therefore, targeted and random biopsies seem essential for accurate surveillance of patients with pre-malignant gastric lesions. A further study in our department concerning the use of random and targeted biopsies demonstrated that nine random biopsies from cardia, corpus, and in particular along the lesser curvature, angulus and antrum are required for optimal detection of pre-malignant gastric lesions in a population at low gastric cancer risk [32]. However, similar to this previous study, in a small percentage of patients in our study, intestinal metaplasia was not confirmed during surveillance endoscopy [32]. Since these patients all underwent endoscopy with extensive biopsy sampling, we assume that the majority, if not all of these patients had a patchy and limited extent of metaplasia and thus a low gastric cancer risk.
Some limitations of this study warrant consideration. First, the endoscopic procedure of WLE and NBI was performed by the same endoscopist. Therefore, detection of intestinal metaplasia and dysplasia by NBI could possibly be biased by the previous white light observations, resulting in an overestimation of the detection rate of NBI. Second, only recently it has been demonstrated that the severity and extent of atrophic gastritis and intestinal metaplasia are adequate predictors of gastric cancer risk [31, 33]. Antrum and angulus were selected in this study because these are the regions of particular interest with the highest prevalence of intestinal metaplasia. Nevertheless, the protocol used in this study is also applicable to the proximal part of the gastric mucosa. A large further study is necessary to confirm that NBI in combination with random biopsy sampling may accurately detect extensive intestinal metaplasia in patients with pre-malignant gastric lesions. Third, although NBI showed an increased detection rate for intestinal metaplasia and dysplasia, it was also related to a higher rate of false positivity than WLE (Table 3). This higher rate does not however imply increasing costs for surveillance, as the decision to embark on surveillance remains dependent on confirmation of endoscopic findings by histology.
In conclusion, NBI considerably increases the diagnostic yield of the detection of advanced premalignant gastric lesions compared to routine WLE. Therefore, NBI seems superior to WLE in the surveillance of patients with these advanced lesions of the gastric mucosa.
Abbreviations
- NBI:
-
Narrow band imaging
- WLE:
-
White light endoscopy
- IM:
-
Intestinal metaplasia
References
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–6740.
de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952.
Lassen A, Hallas J, de Muckadell OB. The risk of missed gastroesophageal cancer diagnoses in users and nonusers of antisecretory medication. Gastroenterology. 2005;129:1179–1186.
Sauerbruch T, Schreiber MA, Schussler P, et al. Endoscopy in the diagnosis of gastritis. Diagnostic value of endoscopic criteria in relation to histological diagnosis. Endoscopy. 1984;16:101–104.
Redeen S, Petersson F, Jonsson KA, et al. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946–950.
Lin BR, Shun CT, Wang TH, et al. Endoscopic diagnosis of intestinal metaplasia of stomach—accuracy judged by histology. Hepatogastroenterology. 1999;46:162–166.
Meshkinpour H, Orlando RA, Arguello JF, et al. Significance of endoscopically visible blood vessels as an index of atrophic gastritis. Am J Gastroenterol. 1979;71:376–379.
Uedo N, Ishihara R, Iishi H, et al. A new method of diagnosing gastric intestinal metaplasia: narrow-band imaging with magnifying endoscopy. Endoscopy. 2006;38:819–824.
Nakayoshi T, Tajiri H, Matsuda K, et al. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080–1084.
Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181.
Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130–131.
Schlemper RJ, Kato Y, Stolte M. Diagnostic criteria for gastrointestinal carcinomas in Japan and Western countries: proposal for a new classification system of gastrointestinal epithelial neoplasia. J Gastroenterol Hepatol. 2000;15(Suppl):G49–G57.
Steyerberg EW. Clinical prediction model: a practical approach to development, validation and updating. New York: Springer; 2009.
Altman DG. ROC curves and confidence intervals: getting them right. Heart. 2000;83:236.
East JE, Tan EK, Bergman JJ, et al. Meta-analysis: narrow band imaging for lesion characterization in the colon, oesophagus, duodenal ampulla and lung. Aliment Pharmacol Ther. 2008;28:854–867.
de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter. 2007;12:1–15.
Ohashi A, Niwa Y, Ohmiya N, et al. Quantitative analysis of the microvascular architecture observed on magnification endoscopy in cancerous and benign gastric lesions. Endoscopy. 2005;37:1215–1219.
Tajiri H, Doi T, Endo H, et al. Routine endoscopy using a magnifying endoscope for gastric cancer diagnosis. Endoscopy. 2002;34:772–777.
Otsuka Y, Niwa Y, Ohmiya N, et al. Usefulness of magnifying endoscopy in the diagnosis of early gastric cancer. Endoscopy. 2004;36:165–169.
Ortner MA, Ebert B, Hein E, et al. Time gated fluorescence spectroscopy in Barrett’s oesophagus. Gut. 2003;52:28–33.
Kato M, Kaise M, Yonezawa J, et al. Autofluorescence endoscopy versus conventional white light endoscopy for the detection of superficial gastric neoplasia: a prospective comparative study. Endoscopy. 2007;39:937–941.
Mayinger B, Jordan M, Horbach T, et al. Evaluation of in vivo endoscopic autofluorescence spectroscopy in gastric cancer. Gastrointest Endosc. 2004;59:191–198.
Shaw D, Blair V, Framp A, et al. Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut. 2005;54:461–468.
Mouzyka S, Fedoseeva A. Chromoendoscopy with hematoxylin in the classification of gastric lesions. Gastric Cancer. 2008;11:15–21. discussion-2.
Zhang JN, Li YQ, Zhao YA, et al. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843–853.
Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol. 2008;24:631–637.
Anagnostopoulos GK, Yao K, Kaye P, et al. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202–207.
Tanaka K, Toyoda H, Kadowaki S, et al. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332–338.
Kaise M, Kato M, Urashima M, et al. Magnifying endoscopy combined with narrow-band imaging for differential diagnosis of superficial depressed gastric lesions. Endoscopy. 2009;41:310–315.
Capelle LG, de Vries AC, Haringsma J, et al. The staging of gastritis with the OLGA system using intestinal metaplasia as accurate alternative for atrophic gastritis. Gastrointest Endosc. 2010.
de Vries AC, Haringsma J, de Vries RA, et al. The use of clinical, histologic, and serologic parameters to predict the intragastric extent of intestinal metaplasia: a recommendation for routine practice. Gastrointest Endosc. 2009;70:18–25.
de Vries AC, Haringsma J, de Vries RA, et al. The yield of endoscopic surveillance of pre-malignant gastric lesions: optimalization of biopsy strategies. 2009 (Submitted).
Rugge M, Genta RM. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807–1808.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Capelle, L.G., Haringsma, J., de Vries, A.C. et al. Narrow Band Imaging for the Detection of Gastric Intestinal Metaplasia and Dysplasia During Surveillance Endoscopy. Dig Dis Sci 55, 3442–3448 (2010). https://doi.org/10.1007/s10620-010-1189-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1189-2