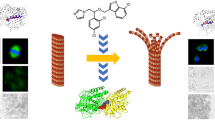
Abstract
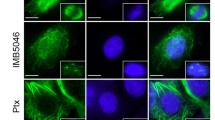
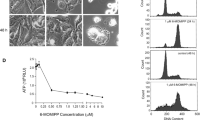
Tioconazole is one of the drugs used to treat topical mycotic infections. It exhibited severe toxicity during systemic administration; however, the molecular mechanism behind the cytotoxic effect was not well established. We employed HeLa cells as a model to investigate the molecular mechanism of its toxicity and discovered that tioconazole inhibited HeLa cell growth through mitotic block (37%). At the half-maximal inhibitory concentration (≈ 15 μM) tioconazole apparently depolymerized microtubules and caused defects in chromosomal congression at the metaphase plate. Tioconazole induced apoptosis and significantly hindered the migration of HeLa cells. Tioconazole bound to goat brain tubulin (Kd, 28.3 ± 0.5 μM) and inhibited the assembly of microtubules in the in vitro assays. We report for the first time that tioconazole binds near to the colchicine site, based on the evidence from in vitro tubulin competition experiment and computational analysis. The conformation of tubulin dimer was found to be “curved” upon binding with tioconazole in the MD simulation. Tioconazole in combination with vinblastine synergistically inhibited the growth of HeLa cells and augmented the percentage of mitotic block by synergistically inhibiting the assembly of microtubules. Our study indicates that the systemic adverse effects of tioconazole are partly due to its effects on microtubules and cell cycle arrest. Since tioconazole is well tolerated at the topical level, it could be developed as a topical anticancer agent in combination with other systemic anticancer drugs.
Graphical abstract







Similar content being viewed by others
Data availability
Additional supporting information may be found online in the Supplementary Information section at the end of this article. All the data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Akuse JT (1984) Assessment of the efficacy of tioconazole (Trosyd) 300 MG ovules in vaginal candidosis: single-dose therapy. Curr Ther Res 36:409–413
Anséhn S, Nilsson L (1984) Direct membrane-damaging effect of ketoconazole and tioconazole on Candida albicans demonstrated by bioluminescent assay of ATP. Antimicrob Agents Chemother 26:22–25
Appadurai P, Rathinasamy K (2014) Indicine N-oxide binds to tubulin at a distinct site and inhibits the assembly of microtubules: a mechanism for its cytotoxic activity. Toxicol Lett 225:66–77
Ashraf SM, Sebastian J, Rathinasamy K (2018) Zerumbone, a cyclic sesquiterpene, exerts antimitotic activity in HeLa cells through tubulin binding and exhibits synergistic activity with vinblastine and paclitaxel. Cell Prolif 52:e12558
Banerjee M, Poddar A, Mitra G, Surolia A, Owa T, Bhattacharyya B (2005) Sulfonamide drugs binding to the colchicine site of tubulin: thermodynamic analysis of the drug− tubulin interactions by isothermal titration calorimetry. J Med Chem 48:547–555
Best RB, Zhu X, Shim J, Lopes PE, Mittal J, Feig M, MacKerell AD Jr (2012) Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J Chem Theory Comput 8:3257–3273
Bhattacharyya B, Wolff J (1974) Promotion of fluorescence upon binding of colchicine to tubulin. Proc Natl Acad Sci 71:2627–2631
Blagosklonny MV (2004) Analysis of FDA approved anticancer drugs reveals the future of cancer therapy. Cell Cycle 3:1033–1040
Borgers M (1980) Mechanism of action of antifungal drugs, with special reference to the imidazole derivatives. Rev Infect Dis 2:520–534
Chou TC, Talalay P (1983) Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci 4:450–454
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzym Regul 22:27–55
Clément MJ, Rathinasamy K, Adjadj E, Toma F, Curmi PA, Panda D (2008) Benomyl and colchicine synergistically inhibit cell proliferation and mitosis: evidence of distinct binding sites for these agents in tubulin. Biochemistry 47:13016–13025
Clissold SP, Heel RC (1986) Tioconazole A review of its antimicrobial activity and therapeutic use in superficial mycoses. Drugs 31:29–51
Crisóstomo-Lucas C, García-Holley P, Hernández-Ortega S, Sánchez-Bartéz F, Gracia-Mora I, Barba-Behrens N (2015) Structural characterization and cytotoxic activity of tioconazole coordination compounds with cobalt (II), copper (II), zinc (II) and cadmium (II). Inorganica Chim Acta 438:245–254
Das A, Choudhury D, Chakrabarty S, Bhattacharya A, Chakrabarti G (2012) Acenaphthenequinone induces cell cycle arrest and mitochondrial apoptosis via disruption of cellular microtubules. Toxicol Res 1:171–185
Dumontet C, Jordan MA (2010) Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov 9:790–803
Fromtling RA (1988) Overview of medically important antifungal azole derivatives. Clin Microbiol Rev 1:187–217
Green RA, Kaplan KB (2003) Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC. The J Cell Biol 163:949–961
Gupta K, Panda D (2002) Perturbation of microtubule polymerization by quercetin through tubulin binding: a novel mechanism of its antiproliferative activity. Biochemistry 41:13029–13038
Gupta K, Bishop J, Peck A, Brown J, Wilson L, Panda D (2004) Antimitotic antifungal compound benomyl inhibits brain microtubule polymerization and dynamics and cancer cell proliferation at mitosis, by binding to a novel site in tubulin. Biochemistry 43:6645–6655
Hamel E, Lin CM (1981) Glutamate-induced polymerization of tubulin: characteristics of the reaction and application to the large-scale purification of tubulin. Arch Biochem Biophys 209:29–40
Jordan MA (2002) Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents 2:1–17
Jordan MA, Kamath K (2007) How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets 7:730–742
Jordan MA, Wilson L (1998) The use and action of drugs in analyzing mitosis. In: Rieder CL (ed) Methods in cell biology. Academic Press, pp 267–295
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79:926–935
Kaur IP, Kakkar S (2010) Topical delivery of antifungal agents. Expert Opin Drug Deliv 7:1303–1327
Kumari R, Kumar R, Open Source Drug Discovery Consortium, Lynn A (2014) g_mmpbsa- a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54:1951–1962
Liu PF, Tsai KL, Hsu CJ, Tsai WL, Cheng JS, Chang HW, Shiau CW, Goan YG, Tseng HH, Wu CH, Reed JC (2018) Drug repurposing screening identifies tioconazole as an ATG4 inhibitor that suppresses autophagy and sensitizes cancer cells to chemotherapy. Theranostics 8:830–845
Lu Y, Chen J, Xiao M, Li W, Miller DD (2012) An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm Res 29:2943–2971
Luduena RF, Roach MC (1991) Tubulin sulfhydryl groups as probes and targets for antimitotic and antimicrotubule agents. Pharmacol Ther 49:133–152
Mahanty S, Raghav D, Rathinasamy K (2021) Vanadocene dichloride induces apoptosis in HeLa cells through depolymerization of microtubules and inhibition of Eg5. J Biol Inorg Chem 26:511–531
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory, New York
Marriott MS, Brammer KW, Faccini J, Faulkner JK, Jevons S, Monro AM, Nachbaur J, Perraud J, Tarbit MH (1983) Tioconazole, a new broad-spectrum antifungal agent: preclinical studies related to vaginal candidiasis. Gynäkol Geburtshilfliche Rundsch 23:1–11
McLoughlin EC, O’Boyle NM (2020) Colchicine-binding site inhibitors from chemistry to clinic: a review. Pharmaceuticals 13:1–43
Mullen P (2004) PARP cleavage as a means of assessing apoptosis. In: Langdon SP (ed) Cancer cell culture. Humana Press, New Jersey, pp 171–181
Müsch A (2004) Microtubule organization and function in epithelial cells. Traffic 5:1–9
Panda D, Rathinasamy K, Santra MK, Wilson L (2005) Kinetic suppression of microtubule dynamic instability by griseofulvin: implications for its possible use in the treatment of cancer. Proc Natl Acad Sci 102:9878–9883
Peng LX, Hsu MT, Bonomi M, Agard DA, Jacobson MP (2014) The free energy profile of tubulin straight-bent conformational changes, with implications for microtubule assembly and drug discovery. PLoS Comput Biol 10:e1003464
Qi C, Wang X, Shen Z, Chen S, Yu H, Williams N, Wang G (2018) Anti-mitotic chemotherapeutics promote apoptosis through TL1A-activated death receptor 3 in cancer cells. Cell Res 28:544–555
Qin SY, Cheng YJ, Lei Q, Zhang AQ, Zhang XZ (2018) Combinational strategy for high-performance cancer chemotherapy. Biomaterials 171:178–197
Rai SS, Wolff J (1996) Localization of the vinblastine-binding site on β-tubulin. J Biol Chem 271:14707–14711
Rathinasamy K, Panda D (2006) Suppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cells. FEBS J 273:4114–4128
Rathinasamy K, Panda D (2008) Kinetic stabilization of microtubule dynamic instability by benomyl increases the nuclear transport of p53. Biochem Pharmacol 76:1669–1680
Sebastian J, Rathinasamy K (2019) Benserazide perturbs Kif15-kinesin binding protein interaction with prolonged metaphase and defects in chromosomal congression: a study based on in silico modeling and cell culture. Mol Inform 39:1900035
Sebastian J, Rathinasamy K (2021) Sertaconazole induced toxicity in HeLa cells through mitotic arrest and inhibition of microtubule assembly. Naunyn-Schmiedeberg's Arch Pharmacol 1–19.
Senapati S, Mahanta AK, Kumar S, Maiti P (2018) Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther 3:1–19
Sobue S, Sekiguchi K (2004) Difference in percutaneous absorption and intracutaneous distribution in guinea pigs among topical antifungal drugs (tioconazole solution, tioconazole cream, miconazole nitrate solution and bifonazole solution). Biol Pharm Bull 27:1428–1432
Tverdek FP, Kofteridis D, Kontoyiannis DP (2016) Antifungal agents and liver toxicity: a complex interaction. Expert Rev Anti Infect Ther 14:765–776
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26:1701–1718
Vanommeslaeghe K, MacKerell AD Jr (2012) Automation of the CHARMM General Force Field (CGenFF) I: bond perception and atom typing. J Chem Inf Model 52:3144–3154
Wilson L, Panda D, Jordan MA (1999) Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct 24:329–335
Acknowledgements
The authors thank NITC and MHRD, Government of India for the financial support in the form of scholarship to Mr. Jomon Sebastian and Infrastructural facilities to Dr. Rathinasamy K.
Funding
Funding was provided by NIT Calicut and Govt. of India.
Author information
Authors and Affiliations
Contributions
JS performed the experiments, analyzed the data and wrote the manuscript. KR designed the experiments, provided the resources for the work, critically analyzed the data and wrote the manuscript. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sebastian, J., Rathinasamy, K. Cytotoxic mechanism of tioconazole involves cell cycle arrest at mitosis through inhibition of microtubule assembly. Cytotechnology 74, 141–162 (2022). https://doi.org/10.1007/s10616-021-00516-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-021-00516-w