Abstract
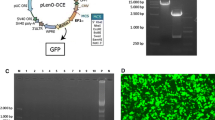
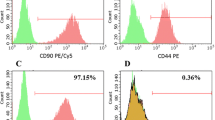
Adipose-derived stem cells (ADSCs) are a type of mesenchymal stem cells with the therapeutic effects that make them one of the best sources for cell therapy. In this study, we aimed to assess the ability of human ADSCs for constant expression of IL-11 and IL-13, simultaneously. In this study, the characterized hADSCs were transduced with a lentiviral vector (PCDH-513B) containing IL-11 and IL-13 genes, and the ability of long-term expression of the transgenes was evaluated by ELISA technique on days 15, 45 and 75 after transduction. Our results indicated a high rate of transduction (more than 90%) in the isolated hADSCs. Our data showed the highest rate of expression on days 75 after transduction which was 242.67 pg/ml for IL-11 and 303.6 pg/ml for IL-13 compared with 35.2 pg/ml and 35.6 pg/ml in untreated cells, respectively (p = 0.001). Besides, MTT assay showed transduction of hADSCs with lentiviral viruses containing IL-11 and IL-13 had no adverse effect on hADSCs proliferation (p-value = 0.89). Finally, we successfully constructed a hADSC population stably overexpressing IL-11 as the neurotrophic cytokine and IL-13 as the anti-inflammatory cytokine and this transduced cells can be used for further studies in EAE mice model.




Similar content being viewed by others
Availability of data and materials
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
Alizadeh A et al (2015) Lentiviral mediated overexpression of NGF in adipose-derived stem cells. Clon Trans 4:3
Association GA (2014) World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am College Dent 81:14
Barde I, Salmon P, Trono D (2010) Production and titration of lentiviral vectors. Curr Protocols Neurosci 53:1–23
Ciuffreda MC et al (2016) Protocols for in vitro differentiation of human mesenchymal stem cells into osteogenic, chondrogenic and adipogenic lineages mesenchymal stem cells. Springer, Berlin, pp 149–158
Colombet J et al (2007) Virioplankton ‘pegylation’: use of PEG (polyethylene glycol) to concentrate and purify viruses in pelagic ecosystems. J Microbiol Methods 71:212–219
Constantin G et al (2009) Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem cells 27:2624–2635
Cuascut FX, Hutton GJ (2019) Stem cell-based therapies for multiple sclerosis: current perspectives. Biomedicines 7:26
Dargahi N et al (2017) Multiple sclerosis: immunopathology and treatment update. Brain Sci 7:78
Dooley D et al (2016) Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports 7:1099–1115
Dulamea A (2015) Mesenchymal stem cells in multiple sclerosis-translation to clinical trials. J Med Life 8:24
Ghasemi N (2015) Therapeutic effects of adipose derived mesenchymal stem cells on remyelination process in inflammatory demyelinating diseases. J Histol Histopathol 2:8
Guan J et al (2015) Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells. Stem Cell Res Ther 6:5
Guglielmetti C et al (2016) Interleukin-13 immune gene therapy prevents CNS inflammation and demyelination via alternative activation of microglia and macrophages. Glia 64:2181–2200
Gurfein BT et al (2009) IL-11 regulates autoimmune demyelination. J Immunol 183:4229–4240
Kimiskidis V, Fassas A (2013) Stem cell-based therapies in multiple sclerosis. J Genet Syndr Gene Ther S 3:2
Klages N, Zufferey R, Trono D (2000) A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther 2:170–176
Kolosowska N et al (2019) Peripheral administration of IL-13 induces anti-inflammatory microglial/macrophage responses and provides neuroprotection in ischemic stroke. Neurotherapeutics 1:16
Liu X, Clark AF, Wordinger RJ (2007) Expression of ciliary neurotrophic factor (CNTF) and its tripartite receptor complex by cells of the human optic nerve head. Mol Vision 13:758
Liu Y et al (2012) Lentiviral-mediated gene transfer into human adipose-derived stem cells: role of NELL1 versus BMP2 in osteogenesis and adipogenesis in vitro. Acta Biochim Biophys Sin 44:856–865
Machado CV, Telles PD, Nascimento IL (2013) Immunological characteristics of mesenchymal stem cells. Revista brasileira de hematologia e hemoterapia 35:62–67
Maheshwari A et al (2013) Local overexpression of interleukin-11 in the central nervous system limits demyelination and enhances remyelination. Mediat Inflamm 2013:685317
Maria AT et al (2017) Adipose-derived mesenchymal stem cells in autoimmune disorders: state of the art and perspectives for systemic sclerosis. Clin Rev Allergy Immunol 52:234–259
Mazini L et al (2019) Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci 20:2523
McLaughlin KA, Wucherpfennig KW (2008) B cells and autoantibodies in the pathogenesis of multiple sclerosis and related inflammatory demyelinating diseases. Adv Immunol 98:121–149
Meyerrose TE et al (2008) Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells 26:1713–1722
Mori S, Maher P, Conti B (2016) Neuroimmunology of the interleukins 13 and 4. Brain Sci 6:18
Münzel EJ, Williams A (2013) Promoting remyelination in multiple sclerosis—recent advances. Drugs 73:2017–2029
Nair A, Frederick TJ, Miller SD (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell Mol Life Sci 65:2702
Ochoa-Repáraz J et al (2008) IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol 181:954–968
Patel J, Balabanov R (2012) Molecular mechanisms of oligodendrocyte injury in multiple sclerosis and experimental autoimmune encephalomyelitis. Int J Mol Sci 13:10647–10659
Payne NL et al (2012) Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adhes Migr 6:179–189
Payne NL et al (2013) Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun 30:103–114
Peferoen L et al (2014) Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 141:302–313
Regmi S et al (2019) Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol 98:5–8
Rostami M, Haidari K, Shahbazi M (2018) The Human IL-23 Decoy Receptor Inhibits T-Cells Producing IL-17 by Genetically Engineered Mesenchymal Stem Cells. Int J Cell Biol. https://doi.org/10.1089/cell.2018.0006
Sochocka M, Diniz BS, Leszek J (2017) Inflammatory response in the CNS: friend or foe? Mol Neurobiol 54:8071–8089
Tavazzi E, Rovaris M, La Mantia L (2014) Drug therapy for multiple sclerosis. CMAJ 186:833–840
Van Vollenstee FA et al (2016) Human adipose derived mesenchymal stromal cells transduced with GFP lentiviral vectors: assessment of immunophenotype and differentiation capacity in vitro. Cytotechnology 68:2049–2060
Vizoso FJ et al (2017) Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci 18:1852
Wang JM et al (2017) Isolation, culture and identification of human adipose-derived stem cells. Exp Ther Med 13:1039–1043
Way SW et al (2015) Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat Commun 6:6532
Winkelmann A et al (2014) Multiple sclerosis treatment and infectious issues: update 2013. Clin Exp Immunol 175:425–438
Zhang Y et al (2006) Interleukin-11 potentiates oligodendrocyte survival and maturation, and myelin formation. J Neurosci 26:12174–12185
Zhang J et al (2011) Promoting myelin repair and return of function in multiple sclerosis. FEBS Lett 585:3813–3820
Zuk PA (2011) Viral transduction of adipose-derived stem cells adipose-derived stem cells. Springer, Berlin, pp 345–357
Funding
This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran (Grant No: 397053).
Author information
Authors and Affiliations
Contributions
AE: Experimental procedures, and preparation of the manuscript; MAA: Experimental procedures, and preparation of the manuscript; MD: Experimental procedures; MA: Experimental procedures; HS: Supervision of the cell culture procedures and study design; MGH: Supervision of the study, data analysis and finalizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study is approved by the ethics committee of the Isfahan University of Medical Sciences, Isfahan, Iran (IR.MUI.MED.REC.1397.026).
Consent to participate
All authors agree to participate in this research study.
Consent for publication
All authors agree to this publication.
Competing interest
There are no conflicts of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eslami, A., Dehbashi, M., Ashja-Arvan, M. et al. Assessment of ability of human adipose derived stem cells for long term overexpression of IL-11 and IL-13 as therapeutic cytokines. Cytotechnology 72, 773–784 (2020). https://doi.org/10.1007/s10616-020-00421-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-020-00421-8