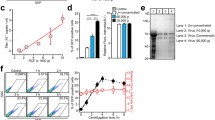
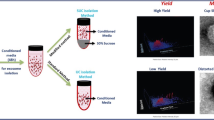
Abstract
In this study, we developed a new purification method using chondroitin sulfate C (CSC) and protamine sulfate (PS) to concentrate lentivirus. To evaluate the efficiency of this new method, we compared it with several previously described purification protocols, including virus concentrated by ultracentrifugation (Ultra), precipitated by polyethylene glycol (PEG), and sedimented by CSC combined with polybrene (PB). After using the different methods to purify and concentrate equivalent amounts of lentivirus supernatant, the virus pellets precipitated by the different methods were resuspended using the equivalent volumes of DMEM. Subsequently, 10 μl of each lentivirus stock carrying EGFP gene was used to transduce two types of cells, human embryonic kidney 293T (HEK293T) cells and mouse mesenchymal stem cells (mMSC). It was obvious that HEK293T and mMSC appeared much intensiver green fluorescence through virus transduction from PS method than from other methods. To quantitate the transduction efficiency of the viruses, we examined virus titer in the cells after transduction using a real-time PCR-based analysis. Accordingly, we verified that PS precipitation could generate virus with a higher titer (4.39 × 108 IU/ml) than PB (2.43 × 108 IU/ml), Ultra (1.16 × 108 IU/ml), and PEG (0.56 × 108 IU/ml) in HEK293T cells. As for HEK293T cells in mMSC, the PS method also generated virus with a higher titer (4.66 × 108 IU/ml) than the Ultra method (2.36 × 108 IU/ml), and a much higher titer than those of the other chemical-based precipitation methods using PB (4.82 × 106 IU/ml) and PEG (8.98 × 104 IU/ml). Furthermore, the HEK293T cells and mMSC transduced by PS(1X)-virus appeared to have higher cell growth ratios, respectively, than the HEK293T cells and mMSC transduced by lentivirus using the other methods. We conclude that our new method for purifying lentivirus is cost-effective, time-saving, and highly efficient, and that lentivirus purification by this means could possibly be used to transduce a variety of cells, including stem cells.




Similar content being viewed by others
References
Armon R, Arella M, Payment P (1988) A highly efficient second-step concentration technique for bacteriophages and enteric viruses using ammonium sulfate and Tween 80. Can J Microbiol 34:651–655
Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK (1993) Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA 90:8033–8037
Costello E, Munoz M, Buetti E, Meylan PR, Diggelmann H, Thali M (2000) Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther 7:596–604
Fontes LV, Campos GS, Beck PA, Brandao CF, Sardi SI (2005) Precipitation of bovine rotavirus by polyethylene [corrected] glycol (PEG) and its application to produce polyclonal and monoclonal antibodies. J Virol Methods 123:147–153
Gatlin J, Melkus MW, Padgett A, Kelly PF, Garcia JV (2001) Engraftment of NOD/SCID mice with human CD34(+) cells transduced by concentrated oncoretroviral vector particles pseudotyped with the feline endogenous retrovirus (RD114) envelope protein. J Virol 75:9995–9999
Han M, Yu D, Song Q, Wang J, Dong P, He J (2015) Polybrene: observations on cochlear hair cell necrosis and minimal lentiviral transduction of cochlear hair cells. Neurosci Lett 600:164–170
Kim BJ, Kim KJ, Kim YH, Lee YA, Kim BG, Cho CM, Kang HR, Kim CG, Ryu BY (2012) Efficient enhancement of lentiviral transduction efficiency in murine spermatogonial stem cells. Mol Cells 33:449–455
Kohno T, Mohan S, Goto T et al (2002) A new improved method for the concentration of HIV-1 infective particles. J Virol Methods 106:167–173
Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A (1991) Simplified mammalian DNA isolation procedure. Nucleic Acids Res 19:4293
Landazuri N, Le Doux JM (2004) Complexation of retroviruses with charged polymers enhances gene transfer by increasing the rate that viruses are delivered to cells. J Gene Med 6:1304–1319
Landazuri N, Le Doux JM (2006) Complexation with chondroitin sulfate C and Polybrene rapidly purifies retrovirus from inhibitors of transduction and substantially enhances gene transfer. Biotechnol Bioeng 93:146–158
Lee H, Song JJ, Kim E, Yun CO, Choi J, Lee B, Kim J, Chang JW, Kim JH (2001) Efficient gene transfer of VSV-G pseudotyped retroviral vector to human brain tumor. Gene Ther 8:268–273
Li GB, Lu GX (2009) Gene delivery efficiency in bone marrow-derived dendritic cells: comparison of four methods and optimization for lentivirus transduction. Mol Biotechnol 43:250–256
Lin P, Correa D, Lin Y, Caplan AI (2011) Polybrene inhibits human mesenchymal stem cell proliferation during lentiviral transduction. PLoS ONE 6:e23891
Lin P, Lin Y, Lennon DP, Correa D, Schluchter M, Caplan AI (2012) Efficient lentiviral transduction of human mesenchymal stem cells that preserves proliferation and differentiation capabilities. Stem Cells Transl Med 1:886–897
McMillin DW, Landazuri N, Gangadharan B, Hewes B, Archer DR, Spencer HT, Le Doux JM (2005) Highly efficient transduction of repopulating bone marrow cells using rapidly concentrated polymer-complexed retrovirus. Biochem Biophys Res Commun 330:768–775
Mizuarai S, Ono K, You J, Kamihira M, Iijima S (2001) Protamine-modified DDAB lipid vesicles promote gene transfer in the presence of serum. J Biochem 129:125–132
Nanba D, Matsushita N, Toki F, Higashiyama S (2013) Efficient expansion of human keratinocyte stem/progenitor cells carrying a transgene with lentiviral vector. Stem Cell Res Ther 4:127
Nasri M, Karimi A, Allahbakhshian Farsani M (2014) Production, purification and titration of a lentivirus-based vector for gene delivery purposes. Cytotechnology 66:1031–1038
Papanikolaou E, Kontostathi G, Drakopoulou E, Georgomanoli M, Stamateris E, Vougas K, Vlahou A, Maloy A, Ware M, Anagnou NP (2013) Characterization and comparative performance of lentiviral vector preparations concentrated by either one-step ultrafiltration or ultracentrifugation. Virus Res 175:1–11
Reiser J (2000) Production and concentration of pseudotyped HIV-1-based gene transfer vectors. Gene Ther 7:910–913
Soleimani M, Nadri S (2009) A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc 4:102–106
Sun G, Xiao J, Wang H, Gong C, Pan Y, Yan S, Wang Y (2014) Efficient purification and concentration of viruses from a large body of high turbidity seawater. MethodsX 1:197–206
Tiscornia G, Singer O, Verma IM (2006) Production and purification of lentiviral vectors. Nat Protoc 1:241–245
Villegas GA, Arguelles MH, Castello AA, Mas NJ, Glikmann G (2002) A rapid method to produce high yields of purified rotavirus particles. J Virol Methods 104:9–19
Yang YW, Hsieh YC (2001) Protamine sulfate enhances the transduction efficiency of recombinant adeno-associated virus-mediated gene delivery. Pharm Res 18:922–927
Zhang B, Xia HQ, Cleghorn G, Gobe G, West M, Wei MQ (2001) A highly efficient and consistent method for harvesting large volumes of high-titre lentiviral vectors. Gene Ther 8:1745–1751
Acknowledgements
This study was supported by grants from the National Science Council, Taiwan (NSC 104-2321-B-415-001-).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, JY., Lee, HH. A new chemical complex can rapidly concentrate lentivirus and significantly enhance gene transduction. Cytotechnology 70, 193–201 (2018). https://doi.org/10.1007/s10616-017-0133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0133-0