Abstract
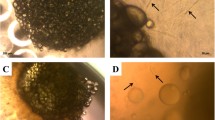
We compared the osteoblastic differentiation abilities of dedifferentiated fat cells (DFATs) and human bone marrow mesenchymal stem cells (hMSCs) as a cell source for bone regeneration therapies. In addition, the utility of DFATs in bone tissue engineering in vitro was assessed by an alpha-tricalcium phosphate (α-TCP)/collagen sponge (CS). Human DFATs were isolated from the submandibular of a patient by ceiling culture. DFATs and hMSCs at passage 3 were cultured in control medium or osteogenic medium (OM) for 14 days. Runx2 gene expression, alkaline phosphatase (ALP) activity, as well as osteocalcin (OCN) and calcium contents were analyzed to evaluate the osteoblastic differentiation ability of both cell types. DFATs seeded in a α-TCP/CS and cultured in OM for 14 days were analyzed by scanning electron microscopy (SEM) and histologically. Compared with hMSCs, DFATs cultured in OM generally underwent superior osteoblastogenesis by higher Runx2 gene expression at all days tested, as well as higher ALP activity at day 3 and 7, OCN expression at day 14, and calcium content at day 7. In SEM analyses, DFATs seeded in a α-TCP/CS were well spread and covered the α-TCP/CS by day 7. In addition, numerous spherical deposits were found to almost completely cover the α-TCP/CS on day 14. Von Kossa staining showed that DFATs differentiated into osteoblasts in the α-TCP/CS and formed cultured bone by deposition of a mineralized extracellular matrix. The combined use of DFATs and an α-TCP/CS may be an attractive option for bone tissue engineering.




Similar content being viewed by others
References
Arima Y, Uemura N, Hashimoto Y, Baba S, Matsumoto N (2013) Evaluation of bone regeneration by porous alpha-tricalcium phosphate/atelocollagen sponge composite in rat calvarial defects. Orthod Waves 72:23–29. doi:10.1016/j.odw.2012.11.001
Canalis E, Giustina A (2001) Glucocorticoid-induced osteoporosis: summary of a workshop. J Clin Endocrinol Metab 86:5681–5685
Colter DC, Class R, DiGirolamo CM, Prockop DJ (2000) Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci USA 97:3213–3218
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Gao Q, Zhao L, Song Z, Yang G (2012) Expression pattern of embryonic stem cell markers in DFAT cells and ADSCs. Mol Biol Rep 39:5791–5804. doi:10.1007/s11033-011-1371-4
Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L, Kawamoto HK, Bradley JP (2007) Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix. J Craniofac Surg 18:895–901
Kishimoto N, Momota Y, Mori R, Hashimoto Y, Imai K, Omasa T, Kotani J (2008) Bone regeneration using dedifferentiated fat cells with PuraMatrixTM. J Oral Tissue Eng 6:127–134
Kishimoto N, Momota Y, Hashimoto Y, Omasa T, Kotani J (2011) Self-assembling peptide RADA16 as a scaffold in bone tissue engineering using dedifferentiated fat cells. J Oral Tissue Engin 8:151–161
Kishimoto N, Momota Y, Hashimoto Y, Ando K, Omasa T, Kotani J (2013) Dedifferentiated fat cells differentiate into osteoblasts in titanium fiber mesh. Cytotechnology 65:15–22. doi:10.1007/s10616-012-9456-z
Lian JB, Stein GS (1992) Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med 3:269–305
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408
Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H (2008) Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 215:210–222. doi:10.1002/jcp.21304
Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M (2010) Long-term expansion and pluripotent marker array analysis of Wharton’s jelly-derived mesenchymal stem cells. Stem Cells Dev 19:117–130
Park KH, Kim H, Moon S, Na K (2009) Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng 108:530–537. doi:10.1016/j.jbiosc.2009.05.021
Patschan D, Loddenkemper K, Buttgereit F (2001) Molecular mechanisms of glucocorticoid-induced osteoporosis. Bone 29:498–505. doi:http://www.ncbi.nlm.nih.gov/pubmed/11728918
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109:656–663. doi:10.1161/01.CIR.0000114522.38265.61
Sakaguchi Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthr Rheum 52:2521–2529
Shimada H, Hashimoto Y, Nakada A, Shigeno K, Nakamura T (2012) Accelerated generation of human induced pluripotent stem cells with retroviral transduction and chemical inhibitors under physiological hypoxia. Biochem Biophys Res Commun 417:659–664
Sierra J, Villagra A, Paredes R, Cruzat F, Gutierrez S, Javed A, Arriagada G, Olate J, Imschenetzky M, Van Wijnen AJ (2003) Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity. Mol Cell Biol 23:3339–3351
Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y (2001) Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288:413–419
Van Den Dolder J, Spauwen PHM, Jansen JA (2003) Evaluation of various seeding techniques for culturing osteogenic cells on titanium fiber mesh. Tissue Eng 9:315–325
Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T (2012) Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res 347:419–427. doi:10.1007/s00441-011-1306-3
Wang J, Ye Y, Tian H, Yang S, Jin X, Tong W, Zhang Y (2011) In vitro osteogenesis of human adipose-derived stem cells by coculture with human umbilical vein endothelial cells. Biochem Biophys Res Commun 412:143–149. doi:10.1016/j.bbrc.2011.07.062
Wiltfang J, Merten HA, Schlegel KA, Schultze-Mosgau S, Kloss FR, Rupprecht S, Kessler P (2002) Degradation characteristics of alpha and beta tri-calcium-phosphate (TCP) in minipigs. J Biomed Mater Res 63:115–121
Yagi K, Kondo D, Okazaki Y, Kano K (2004) A novel preadipocyte cell line established from mouse adult mature adipocytes. Biochem Biophys Res Commun 321:967–974
Yamada Y, Ueda M, Naiki T, Takahashi M, Hata KI, Nagasaka T (2004) Autogenous injectable bone for regeneration with mesenchymal stem cells and platelet-rich plasma: tissue-engineered bone regeneration. Tissue Eng 10:955–964
Zhao J, Shinkai M, Takezawa T, Ohba S, Chung UI, Nagamune T (2009) Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J Biosci Bioeng 107:318–323. doi:10.1016/j.jbiosc.2008.10.007
Zhu Y, Liu T, Song K, Fan X, Ma X, Cui Z (2008) Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct 26:664–675
Zhu H, Schulz J, Schliephake H (2010) Human bone marrow stroma stem cell distribution in calcium carbonate scaffolds using two different seeding methods. Clin Oral Implants Res 21:182–188
Acknowledgments
We thank Dr. Noboru Sasaki (Department of Oral and Maxillofacial Surgery, Amagasaki Chuo Hospital) for his cooperation with collecting the adipose tissue. This study was supported by a Grant-in-Aid for Scientific Research (B) No. 24792263 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakamoto, F., Hashimoto, Y., Kishimoto, N. et al. The utility of human dedifferentiated fat cells in bone tissue engineering in vitro. Cytotechnology 67, 75–84 (2015). https://doi.org/10.1007/s10616-013-9659-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-013-9659-y