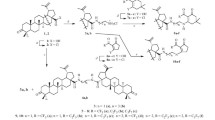
New potentially biologically active amidoethanesulfonamides of betulonic acid were synthesized by the acid chloride method via conjugation of betulonic acid with 2-aminoethanesulfonamides as the free bases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pentacyclic lupane-type triterpenoids form a promising platform for development of biologically active compounds with various types and mechanisms of antiviral activity [1], including against influenza viruses [2, 3], hepatitis B and D [4], human immunodeficiency [5,6,7], Dengue and Chikungunya flaviviruses [8], and corona viruses HCoV-229E [9] and SARS-CoV-2 [10]. Currently, expansion of the library of modified triterpenes and identification among them of novel antiviral agents are becoming especially critical. One approach to the synthesis of antiviral compounds consists of introducing a sulfonamide group into a triterpenoid structure. The sulfonamide group is the basis of various antimicrobial, antitumor, anti-inflammatory, antiviral, and many other medicines and biologically active compounds [11,12,13]. HIV-1 maturation inhibitors were observed among derivatives of lupane triterpenoids with a C-3 or C-28 sulfonamide group [14, 15].
In continuation of research on the modification of lupane triterpenoids to develop biologically active compounds, new sulfonamide derivatives of betulonic acid (1) were synthesized by us. The sulfonamide group was bonded to the triterpene skeleton through an amidoethane spacer. Previously, sulfonamides of this series were first prepared in low yields by us using the reaction of betulinic and betulonic acids with 2-aminoethanesulfonamide hydrochlorides in the presence of Mukaiyama reagent [16].
The present article demonstrates the syntheses of amidoethanesulfonamides of betulonic acid 2a–d via the acid chloride method using 2-aminoethanesulfonamides 3a–d as the free bases. Compounds 3a–d were obtained in 30–51% yields from phthalic anhydride and 2-aminoethanesulfonic acid (taurine) through 2-phthalimidoethanesulfonyl chloride (4) and 2-phthalimidoethanesulfonamides 5a–d using the literature method [17]. The phthalyl protection of the amine in 5a–d was removed by hydrazine hydrate upon refluxing in EtOH. The target amidoethanesulfonamides 2a–d were obtained in 49–78% yields after purification by column chromatography over silica gel.
The structures of conjugates 2a–d were confirmed by 1D and 2D NMR spectroscopy (1H, 13C {1H}, 13C DEPT, 1H–1H COSY, 1H–13C HSQC, 1H–13C HMBC, 1H−15N HSQC, 1H−15N HMBC) and high-resolution mass spectrometry. Resonances in PMR and 13C NMR spectra of all synthesized sulfonamides 2a–d were fully assigned. Characteristic resonances of NH(CH2)2SO2 in proton spectra included a quartet at 3.71–3.75 ppm (2H-2′′) and a multiplet at 3.00–3.05 (2H-1′′, for 2a, c, d) or two doublets of triplets for diastereotopic protons H-1a′′ and H-1b′′ at 3.22 and 3.29 ppm (for 2b). 13C NMR spectra showed resonances for C-2′′ at 33.56–33.95 ppm while the C-1′′ chemical shifts varied from 43.12 to 51.46 ppm, depending on the amine substituent on the S atom.

Experimental
1H and 13C NMR spectra were recorded using TMS as an internal standard or NH3 (for 15N) as an external standard on an Avance III-500 (Bruker, Germany) at operating frequency 500.13 MHz (1H), 125.47 MHz (13C), and 50.68 MHz (15N) or AMXIII-300 spectrometer (Bruker, Germany) at operating frequency 300.13 MHz (1H) and 75.47 MHz (13C). Positive-ion mass spectra were measured on an Agilent 1260 Infinity II/6530-LC/Q-TOF high-resolution mass spectrometer or an LCMS-2010EV liquid chromatograph-mass spectrometer (Shimadzu). Rotation angles were measured on a PerkinElmer 341C instrument. Column chromatography used SiO2 (L grade, 40/60 μm, Russia). TLC used Sorbfil plates (OOO Imid, Krasnodar, Russia). Chromatograms were detected in a thin layer using anisaldehyde, I2, and UV light. Melting points were determined on a Boetius instrument (Germany). 2-Phthalimidoethanesulfonamides 5a and 5b were prepared by the literature methods [18 and 17, respectively].
Preparation of 5c and 5d. A suspension of sulfonyl chloride 4 (1.827 mmol) in anhydrous CH2Cl2 (10 mL) was treated with the appropriate amine (1.827 mmol) and Et3N (3.654 mmol), stirred for 5 h, left overnight, and treated with H2O (10 mL). The organic layer was separated. The aqueous layer was extracted with CH2Cl2. The organic layer was washed with saturated NaCl solution, dried over Na2SO4, and evaporated to afford 5c and 5d.
2-[2-(4-Benzylpiperidin-1-ylsulfonyl)ethyl]isoindoline-1,3-dione (5c), yield 88%. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 1.29 (2H, m, Ha-3a′, 5a′), 1.50–1.93 (3H, m, H-3b′, 5b′, H-4′), 2.54 (2H, d, J = 6.6, H-7′), 2.75 (2H, t, J = 11.8, H-2a′, 6a′), 3.31 (2H, t, J = 6.8, H-1′′), 3.78 (2H, d, J = 11.8, H-2b′, 6b′), 4.12 (2H, t, J = 6.8, H-2′′), 7.14 (2H, d, J = 7.3, Ph: H-2, 6), 7.23 (1H, t, J = 7.3, Ph: H-4), 7.27 (2H, m, Ph: H-3, 5), 7.75 (2H, m, Ar), 7.87 (2H, m, Ar).
2-[2-(4-Methylpiperazin-1-ylsulfonyl)ethyl]isoindoline-1,3-dione (5d), yield 70 %. 1H NMR (300 MHz, CDCl3, δ, ppm, J/Hz): 2.35 (3H, s, H-7′), 2.54 (4H, m, 2H-3′, 5′), 3.37 (6H, m, 2H-1′′, 2H-2′, 6′), 4.14 (2H, m, H-2′′), 7.74 (2H, m, Ar), 7.87 (2H, m, Ar). APCI-MS m/z 338.1 [M + H]+ (calcd for C15H20N3O4S, 338.1).
Preparation of 2-Aminoethanesulfonamides 3a–d. A suspension of the appropriate 2-phthalimidoethanesulfonamide 5a–d (1.189 mmol) was dissolved with heating in EtOH (10 mL), treated with N2H4·H2O (1.43 mmol), refluxed for 8–15 h until a voluminous white precipitate formed, and cooled. The precipitate was filtered off. The filtrate was evaporated. The solid was dissolved in CHCl3 or MTBE (for 3c). The insoluble precipitate was filtered off. The filtrate was evaporated to afford 3a–d.
2-Amino-N-butylethanesulfonamide (3a), yield 37%. The 1H NMR spectrum agreed with the literature [18].
2-Amino-N-phenylethanesulfonamide (3b), yield 51%. The 1H NMR spectrum agreed with the literature [19].
2-(4-Benzylpiperidin-1-ylsulfonyl)ethanamine (3c), yield 45%. 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 1.29 (2H, m, Ha-3a′, 5a′), 1.58–1.77 (3H, m, H-3b′, 5b′, H-4′), 2.53 (2H, m, NH2), 2.55 (2H, d, J = 7.2, H-7′), 2.67 (2H, t, J = 12.0, H-2a′, 6a′), 2.99 (2H, m, H-1′′), 3.16 (2H, m, H-2′′), 3.76 (2H, d, J = 12.0, H-2b′, 6b′), 7.12 (2H, d, J = 7.4, Ph: H-2, 6), 7.19 (1H, t, J = 7.4, Ph: H-4), 7.26 (1H, t, J = 7.4, Ph: H-3*), 7.27 (1H, t, J = 7.4, Ph: H-5*). 13C NMR (125 MHz, CDCl3, δ, ppm): 31.72 (C-3′, 5′), 36.61 (C-2′′), 37.52 (C-4′), 42.70 (C-1′′), 45.97 (C-2′, 6′), 51.96 (C-7′), 126.09 (Ph: C-4), 128.30 (Ph: C-3, 5), 129.02 (Ph: C-2, 6), 139.69 (Ph: C-1).
2-(4-Methylpiperazin-1-ylsulfonyl)ethanamine (3d), yield 30%. 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 1.95 (2H, br.s, NH2), 2.31 (3H, s, H-7′), 2.48 (4H, t, J = 4.3, H-3′, 5′), 3.03 (2H, t, J = 6.1, H-1′′), 3.19 (2H, t, J = 6.1, H-2′′), 3.29 (4H, t, J = 4.3, H-2′, 6′). 13C NMR (125 MHz, CDCl3, δ, ppm): 36.49 (C-2′′), 45.55 (C-2′, 6′), 45.87 (C-7′), 51.73 (C-1′′), 54.48 (C-3′, 5′).
General Method for Preparing Conjugates 2a–d. A solution of acid 1 (0.229 mmol) in anhydrous CH2Cl2 (5 mL) was treated with (COCl)2 (0.2 mL, 2.290 mmol), stirred for 2 h, and evaporated. The resulting betulonic acid chloride was dissolved in anhydrous CH2Cl2 (5 mL), treated with the appropriate sulfonamide (3a–d, 0.344 mmol) in anhydrous CH2Cl2 (3 mL) and Et3N (0.10 mL, 0.687 mmol), stirred for 4 h, left overnight, and evaporated. The solid was chromatographed over SiO2 (hexane–EtOAc, 5:1, 3:1, 2:1).
N-[2-(N-Butylsulfamoyl)ethyl]-3-oxolup-20(29)-ene-17β-carboxamide (2a), yield 61%, mp 97–99°C, \({\left[\mathrm{\alpha }\right]}_{\mathrm{D}}^{20}\) +20.5° (c 0.43, CH2Cl2). 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 0.92, 1.01, 1.06, 1.68 (3H each, s, CH3-25*, 24, 23, 30), 0.97 (6H, s, CH3-26*, 27), 0.94 (3H, t, J = 7.4, H-4′), 1.04 (1H, m, H-12a), 1.17 (1H, ddd, J = 13.3, 2.8, H-15a), 1.24–1.50 (14H, m, H-1a, 21a, 16a, 15b, 2H-6, 7, 11, H-5, 9, 2H-3′), 1.54 (1H, dd, J = 13.5, 2.6, H-22a), 1.57 (3H, m, H-18, H-2′), 1.73 (1H, dd, J = 11.2, 2.0, H-12b), 1.79 (1H, ddd, J = 12.2, 8.5, 1.9, H-16b), 1.85–1.95 (2H, m, H-1b, 21b), 1.98 (1H, dt, J = 13.5, 3.0, H-22b), 2.40 (1H, ddd, J = 15.6, 7.5, 4.4, H-2a), 2.48 (2H, m, H-2b, 13), 3.10 (1H, td, J = 11.2, 4.3, H-19), 3.05–3.25 (4H, m, H-1′, 1′′), 3.72 (2H, q, J = 5.3, H-2′′), 4.59, 4.73 (1H each, s, H-29), 4.70 (1H, br.s, NHBu), 6.47 (1H, t, J = 5.8, NH). 13C NMR (125 MHz, CDCl3, δ, ppm): 13.63 (C-4′), 14.57 (C-27), 15.99 (C-25, 26), 19.46 (C-30), 19.66 (C-6), 19.78 (C-3′), 21.04 (C-24), 21.49 (C-11), 25.62 (C-12), 26.66 (C-23), 29.39 (C-15), 30.82 (C-21), 32.37 (C-2′), 33.14 (C-16), 33.38 (C-22), 33.68 (C-7), 33.95 (C-2′′), 34.17 (C-2), 36.93 (C-10), 37.82 (C-13), 39.64 (C-1), 40.73 (C-8), 42.52 (C-14), 43.12 (C-1′), 46.71 (C-19), 47.35 (C-4), 49.97 (C-9, 18), 52.18 (C-1′′), 54.97 (C-5), 55.71 (C-17), 109.53 (C-29), 150.75 (C-20), 176.70 (C-28), 218.37 (C-3). 15N (50.68 MHz, CDCl3, δ, ppm): 93.74 (NHBu), 105.94 (C(O)NH). HR-ESI-MS m/z 617.4351 [M + H]+ (calcd for C36H61N2O4S, 617.4347).
N-[2-(N-Phenylsulfamoyl)ethyl]-3-oxolup-20(29)-ene-17β-carboxamide (2b), yield 78%, mp 125–127°C, \({\left[\mathrm{\alpha }\right]}_{\mathrm{D}}^{20}\) +12.7° (c 0.83, CH2Cl2). 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 0.95, 1.02, 1.07, 1.66 (3H each, s, CH3-27, 24, 23, 30), 0.89 (6H, s, CH3-25, 26), 1.00 (1H, dd, J = 11.2, 4.6, H-12a), 1.13 (1H, dt, J = 13.5, 2.9, H-15a), 1.18–1.56 (12H, m, H-1a, 21a, 16a, H-15b, 2H-6, 7, 11, H-5, 9), 1.52 (1H, dd, J = 13.4, 2.9, H-22a), 1.56 (1H, t, J = 11.3, H-18), 1.70 (1H, dd, J = 11.2, 2.8, H-12b), 1.76 (1H, ddd, J = 12.2, 8.2, 1.8, H-16b), 1.82–1.91 (2H, m, H-1b, 21b), 1.96 (1H, dt, J = 13.6, 2.7, H-22b), 2.39 (1H, ddd, J = 15.5, 7.5, 4.3, H-2a), 2.47 (2H, m, H-2b, 13), 3.05 (1H, td, J = 11.2, 4.2, H-19), 3.22 (1H, dt, J = 14.5, 5.6, H-1a′′), 3.29 (1H, dt, J = 14.5, 5.6, H-1b′′), 3.75 (2H, q, J = 5.6, H-2′′), 4.58, 4.70 (1H each, s, H-29), 6.42 (1H, t, J = 5.9, NH), 7.54 (1H, s, NHPh), 7.18 (1H, t, J = 7.3, Ph: H-4), 7.28 (2H, d, J = 7.3, Ph: H-2, 6), 7.34 (2H, t, J = 7.3, Ph: H-3, 5). 13C NMR (125 MHz, CDCl3, δ, ppm): 14.54 (C-27), 15.86 (C-26*), 15.97 (C-25*), 19.44 (C-30), 19.65 (C-6), 21.04 (C-24), 21.44 (C-11), 25.60 (C-12), 26.66 (C-23), 29.36 (C-15), 30.76 (C-21), 33.39 (C-22), 33.63 (C-2′′), 33.78 (C-7), 34.17 (C-2), 36.92 (C-10), 37.36 (C-13), 38.09 (C-16), 39.63 (C-1), 40.71 (C-8), 42.49 (C-14), 46.64 (C-19), 47.35 (C-4), 49.97 (C-9, 18), 51.46 (C-1′′), 54.99 (C-5), 55.71 (C-17), 109.51 (C-29), 121.28 (Ph: C-2, 6), 125.56 (Ph: C-4), 129.69 (Ph: C-3, 5), 136.59 (Ph: C-1), 150.67 (C-20), 176.93 (C-28), 218.50 (C-3). 15N (50.68 MHz, CDCl3, δ, ppm): 104.40 (C(O)NH), 115.74 (NHPh). HR-ESI-MS m/z 637.4041 [M + H]+ (calcd for C38H57N2O4S, 637.4034).
N-[2-(4-Benzylpiperidin-1-ylsulfonyl)ethyl]-3-oxolup-20(29)-ene-17β-carboxamide (2c), yield 49%, mp 108–110°C, \({\left[\mathrm{\alpha }\right]}_{\mathrm{D}}^{20}\) +12.6° (c 0.49, CH2Cl2). 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 0.89, 0.95, 0.96, 1.00, 1.05, 1.66 (3H each, s, CH3-26*, 27, 25*, 24, 23, 30), 1.04 (1H, m, H-12a), 1.17 (1H, dt, J = 13.4, 2.8, H-15a), 1.23–1.48 (14H, m, H-1a, 16a, 21a, H-15b, 2H-6, 7, 11, H-5, 9, H-3a′, 5a′), 1.51 (1H, dd, J = 13.4, 3.1, H-22a), 1.56 (1H, t, J = 11.0, H-18), 1.65 (1H, m, H-4′), 1.69 (2H, m, H-3b′, 5b′), 1.71 (1H, dd, J = 11.3, 2.8, H-12b), 1.78 (1H, ddd, J = 12.3, 8.5, 1.9, H-16b), 1.84–1.94 (2H, m, H-1b, 21b), 1.98 (1H, dt, J = 13.4, 3.1, H-22b), 2.38 (1H, ddd, J = 15.4, 7.5, 4.4, H-2a), 2.46 (2H, m, H-2b, 13), 2.56 (2H, d, J = 7.2, H-7′), 2.68 (2H, t, J = 12.4, H-2a′, 6a′), 3.00 (2H, m, H-1′′), 3.07 (1H, td, J = 11.0, 4.2, H-19), 3.71 (2H, q, J = 5.2, H-2′′), 3.74 (2H, td, J = 12.4, 3.1, H-2b′, 6b′), 4.5, 4.72 (1H each, s, H-29), 6.43 (1H, t, J = 5.7, NH), 7.12 (2H, d, J = 7.3, Ph: H-2, 6), 7.19 (1H, t, J = 7.3, Ph: H-4), 7.28 (2H, t, J = 7.3, Ph: H-3, 5). 13C NMR (125 MHz, CDCl3, δ, ppm): 14.55 (C-27), 15.97 (C-26*), 16.00 (C-25*), 19.43 (C-30), 19.64 (C-6), 21.04 (C-24), 21.48 (C-11), 25.60 (C-12), 26.64 (C-23), 29.40 (C-15), 30.80 (C-21), 31.73 (C-3′), 31.74 (C-5′), 33.35 (C-22), 33.56 (C-2′′), 33.69 (C-7), 34.14 (C-2), 36.91 (C-10), 37.49 (C-4'), 37.80 (C-13), 38.11 (C-16), 39.62 (C-1), 40.71 (C-8), 42.51 (C-14), 42.71 (C-7′), 46.05 (C-2′, 6′), 46.69 (C-19), 47.32 (C-4), 48.61 (C-1′′), 49.90 (C-18), 49.96 (C-9), 54.96 (C-5), 55.71 (C-17), 109.51 (C-29), 126.19 (Ph: C-4), 128.32 (Ph: C-3, 5), 129.05 (Ph: C-2, 6), 139.61 (Ph: C-1), 150.75 (C-20), 176.56 (C-28), 218.15 (C-3). 15N (50.68 MHz, CDCl3, δ, ppm): 105.20 (C(O)NH). HR-ESI-MS m/z 719.4825 [M + H]+ (calcd for C44H67N2O4S, 719.4816).
N-[2-(4-Methylpiperazin-1-ylsulfonyl)ethyl]-3-oxolup-20(29)-ene-17β-carboxamide (2d), yield 64%, mp118–119°C, \({\left[\mathrm{\alpha }\right]}_{\mathrm{D}}^{20}\) +17.5° (c 0.5, CH2Cl2). 1H NMR (500 MHz, CDCl3, δ, ppm, J/Hz): 0.91, 0.95, 0.96, 1.00, 1.05, 1.67 (3H each, s, CH3-26, 27, 25, 24, 23, 30), 1.01 (1H, m, H-1a), 1.16 (1H, dt, J = 13.3, 2.8, H-15a), 1.23–1.47 (12H, m, H-1a, 16a, 21a, H-15b, 2H-6, 7, 11, H-5, 9), 1.52 (1H, dd, J = 13.3, 3.1, H-22a), 1.57 (1H, t, J = 11.3, H-18), 1.71 (1H, dd, J = 11.1, 2.5, H-12b), 1.79 (1H, ddd, J = 12.2, 8.6, 1.9, H-16b), 1.85–1.95 (2H, m, H-1b, 21b), 1.97 (1H, dt, J = 13.5, 3.1, H-22b), 2.33 (3H, s, H-7′), 2.38 (1H, ddd, J = 15.5, 7.4, 4.3, H-2a), 2.46 (2H, m, H-2b, 13), 2.50 (4H, t, J = 3.5, H-2′, 6′), 3.04 (2H, m, H-1′′), 3.07 (1H, td, J = 11.2, 4.4, H-19), 3.30 (4H, t, J = 3.5, H-3′, 5′), 3.73 (2H, q, J = 5.8, H-2′′), 4.58, 4.72 (1H each, s, H-29), 6.39 (1H, t, J = 5.8, NH). 13C NMR (125 MHz, CDCl3, δ, ppm): 14.53 (C-27), 15.94 (C-26), 16.00 (C-25), 19.43 (C-30), 19.63 (C-6), 21.02 (C-24), 21.46 (C-11), 25.60 (C-12), 26.62 (C-23), 29.39 (C-15), 30.79 (C-21)*, 33.37 (C-22)*, 33.48 (C-7), 33.70 (C-2′′), 34.13 (C-2), 36.92 (C-10), 37.79 (C-13), 38.08 (C-16), 39.62 (C-1), 40.72 (C-8), 42.51 (C-14), 45.50 (C-2′, 6′), 45.80 (C-7′), 46.67 (C-19), 47.32 (C-4), 48.32 (C-1′′), 49.91 (C-18), 49.97 (C-9), 54.37 (C-5), 54.98 (C-3′, 5′), 55.71 (C-17), 109.51 (C-29), 150.72 (C-20), 176.55 (C-28), 218.13 (C-3). 15N (50.68 MHz, CDCl3, δ, ppm): 35.60 (N-4′), 91.90 (N-1′), 105.50 (C(O)NH). HR-ESI-MS m/z 644.4464 [M + H]+ (calcd for C37H62N3O4S, 644.4456).
Resonances of atoms in 1H and 13C NMR spectra marked with an “*” could change places.
References
S. Xiao, Z. Tian, Y. Wang, L. Si, L. Zhang, and D. Zhou, Med. Res. Rev., 38, 951 (2018).
Y. Chen, X. Wang, Y. Zhu, L. Si, B. Zhang, Y. Zhang, L. Zhang, D. Zhou, and S. Xiao, Mol. Pharmaceutics, 17 (7), 2546 (2020).
H. Wang, R. Xu, Y. Shi, L. Si, P. Jiao, Z. Fan, X. Han, X. Wu, X. Zhou, F. Yu, Y. Zhang, L. Zhang, L. Zhang, D. Zhou, and S. Xiao, Eur. J. Med. Chem., 110, 376 (2016).
M. Kirstgen, K. A. A. T. Lowjaga, S. F. Muller, N. Goldmann, F. Lehmann, S. Alakurtti, J. Yli-Kauhaluoma, D. Glebe, and J. Geyer, Sci. Rep., 10, 21772 (2020).
I. Dicker, J. L. Jeffrey, T. Protack, Z. Lin, M. Cockett, Y. Chen, S.-Y. Sit, M. Gartland, N. A. Meanwell, A. Regueiro-Ren, D. Drexler, J. Cantone, B. McAuliffe, and M. Krystal, Antimicrob. Agents Chemother., 66 (1), 1876 (2022).
A. Regueiro-Ren, S.-Y. Sit, Y. Chen, J. Chen, J. J. Swidorski, Z. Liu, B. L. Venables, N. Sin, R. A. Hartz, T. Protack, Z. Lin, S. Zhang, Z. Li, D.-R. Wu, P. Li, J. Kempson, X. Hou, A. Gupta, R. Rampulla, A. Mathur, H. Park, A. Sarjeant, Y. Benitex, S. Rahematpura, D. Parker, T. Phillips, R. Haskell, S. Jenkins, K. S. Santone, M. Cockett, U. Hanumegowda, I. Dicker, N. A. Meanwell, and M. Krystal, J. Med. Chem., 65, 11927 (2022).
Q. Wang, Y. Li, L. Zheng, X. Huang, Y. Wang, C.-H. Chen, Y.-Y. Cheng, S. L. Morris-Natschke, and K.-Hsiung Lee, ACS Med. Chem. Lett., 11 (11), 2290 (2020).
M. W. C. Loe, E. Hao, M. Chen, C. Li, R. C. H. Lee, I. X. Y. Zhu, Z. Y. Teo, W.-X. Chin, X. Hou, J. Deng, and J. J. H. Chu, Antiviral Res., 184, 104954 (2020).
A. Stevaert, B. Krasniqi, B. Van Loy, T. Nguyen, J. Thomas, J. Vandeput, D. Jochmans, V. Thiel, R. Dijkman, W. Dehaen, A. Voet, and L. Naesens, J. Med. Chem., 64, 5632 (2021).
M. Kadela-Tomanek, M. Jastrzebska, K. Marciniec, E. Bebenek, E. Chrobak, and S. Boryczka, Crystals, 11, 76 (2021).
C. Zhao, K. P. Rakesh, L. Ravidar, W.-Y. Fang, and H.-L. Qin, Eur. J. Med. Chem., 162, 679 (2019).
S. Apaydin and M. Torok, Bioorg. Med. Chem. Lett., 29, 2042 (2019).
A. K. Timiri, S. Selvarasu, M. Kesherwani, V. Vishwanathan, B. N. Sinha, D. Velmurugan, and V. Jayaprakash, Bioorg. Chem., 62, 74 (2015).
N. Sin, Z. Liu, et al., US Pat. Appl. 20130296554A1, Nov. 7, 2013.
A. Reguerio-Ren, J. Swidorski, et al., US Pat. 8,754,068 B2, Jun. 17, 2014.
N. G. Komissarova, S. N. Dubovitskii, O. V. Shitikova, and A. V. Orlov, Chem. Nat. Compd., 57, 712 (2021).
R. A. Smits, M. Adami, E. P. Istyastono, O. P. Zuiderveld, C. M. E. van Dam, F. J. J. de Kanter, A. Jongejan, G. Coruzzi, R. Leurs, and I. J. P. de Esch, J. Med. Chem., 53 (6), 2390 (2010).
R. Goschke, S. Stutz, V. Rasetti, N.-C. Cohen, J. Rahuel, P. Rigollier, H.-P. Baum, P. Forgiarini, C. R. Schnell, T. Wagner, M. G. Gruetter, W. Fuhrer, W. Schilling, F. Cumin, J. M. Wood, and J. Maibaum, J. Med. Chem., 50 (20), 4818 (2007).
T. Sugimoto, N. Sasamoto, et al., WO Pat. 2012115256A1, Aug. 30, 2012.
Acknowledgment
The work was supported by Russian Science Foundation Grant No. 22-43-08002. The spectral part of the work used equipment at the Khimiya CCU, UfIC, UfFRC, RAS, and Agidel CCU, UfFRC, RAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 2, March–April, 2023, pp. 266–269.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Komissarova, N.G., Orlov, A.V. & Spirikhin, L.V. Synthesis of New Amidoethanesulfonamides of Betulonic Acid. Chem Nat Compd 59, 313–317 (2023). https://doi.org/10.1007/s10600-023-03983-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-03983-z