A 2,3-secotriterpenoid with a methylketone was prepared from 1-hydroxyiminodihydrobetulonic acid methyl ester via a Grignard reaction followed by a Beckmann rearrangement. Functionalization of it led to the formation of 3-hydroxy- and 31-bromo-substituted derivatives; intramolecular cyclization, to the formation of a five-membered ring A with an alkene-nitrile. The synthetic products included compounds (5a and 9) with moderate cytotoxicity (IC50 25.22–46.66 μM) against MCF-7, HCT116, A549, and PC-3 cancer cells.

Similar content being viewed by others
References
S. Xiao, Z. Tian, Y. Wang, L. Si, L. Zhang, and D. Zhou, Med. Res. Rev., 38, 951 (2018).
A. Hordyjewska, A. Ostapiuk, A. Horecka, and J. Kurzepa, Phytochem. Rev., 18, 929 (2019).
S. Amiri, S. Dastghaib, M. Ahmadi, P. Mehrbod, F. Khadem, H. Behrooj, and S. Ghavami, Biotechnol. Adv., 38, 107409 (2020).
A. Lombrea, A. D. Scurtu, S. Avram, I. Z. Pavel, M. Turks, J. Lugioina, and C. Danciu, Int. J. Mol. Sci., 22 (7), 3676 (2021).
P. A. Krasutsky, Nat. Prod. Rep., 23, 919 (2006).
L. A. Baltina, O. B. Flekhter, L. R. Nigmatullina, E. I. Boreko, N. I. Pavlova, S. N. Nikolaeva, O. V. Savinova, and G. A. Tolstikov, Bioorg. Med. Chem. Lett., 13, 3549 (2003).
R. Csuk, K. Schmuck, and R. Schafer, Tetrahedron Lett., 47, 8769 (2006).
S. A. Popov, L. P. Kozlova, L. M. Kornaukhova, and A. V. Shpatov, Ind. Crops. Prod., 92, 197 (2016).
A. V. Pereslavtseva, I. A. Tolmacheva, P. A. Slepukhin, O. S. El′tsov, I. I. Kucherov, V. F. Eremin, and V. V. Grishko, Chem. Nat. Compd., 49, 1059 (2014).
A. V. Konysheva, V. O. Nebogatikov, I. A. Tolmacheva, M. V. Dmitriev, and V. V. Grishko, Eur. J. Med. Chem., 140, 74 (2017).
A. V. Konysheva, A. E. Zhukova, M. V. Dmitriev, and V. V. Grishko, Chem. Nat. Compd., 54, 1094 (2018).
D. V. Eroshenko, G. F. Krainova, A. V. Konysheva, M. V. Dmitriev, and V. V. Grishko, Bioorg. Med. Chem. Lett., 28, 3752 (2018).
A. V. Konysheva, D. V. Eroshenko, and V. V. Grishko, Nat. Prod. Commun., 14 (10), 1 (2019).
E. Y. Rybalkina, N. I. Moiseeva, A. F. Karamysheva, D. V. Eroshenko, A. V. Konysheva, A. V. Nazarov,
and V. V. Grishko, Chem. Biol. Interact., 348, 109645 (2021).
15. J. Pokorny, S. Krajcovicova, M. Hajduch, M. Holoubek, S. Gurska, P. Dzubak, T. Volna, I. Popa, and M. Urban, Future Med. Chem., 10 (5), 483 (2018).
16. G. F. Krainova, O. N. Gagarskikh, and V. V. Grishko, Chem. Nat. Compd., 58, 693 (2022).
R. Csuk, C. Nitsche, R. Sczepek, S. Schwarz, and B. Siewer, Arch. Pharm., Chem. Life Sci., 346, 232 (2013).
18. G. C. Souza, Jr., G. C. Franchi, A. E. Nowill, L. S. Santos, C. N. Alves, L. E. S. Barata, and C. K. Z. Andrade, J. Braz. Chem. Soc., 28 (11), 2229 (2017).
19. A. V. Konysheva, I. A. Tolmacheva, D. V. Eroshenko, and V. V. Grishko, Chem. Nat. Compd., 53, 497 (2017).
G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 64, 112 (2008).
G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 71, 3 (2015).
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, and H. Puschmann, J. Appl. Crystallogr., 42, 339 (2009).
B. Keil, Laboratoriumstechnik der organische Chemie, Akademie-Verlag, Berlin (1961) [Russian translation, Mir, Moscow, 1966, 591 pp].
I. Y. Strobykina, B. F. Garifullin, R. R. Sharipova, A. D. Voloshina, A. S. Strobykina, A. B. Dobrynin, and V. E. Kataev, Chem. Nat. Compd., 53, 1101 (2017).
I. A. Tolmacheva, A. V. Nazarov, O. A. Maiorova, and V. V. Grishko, Chem. Nat. Compd., 44, 606 (2008).
T. Mosmann, J. Immunol. Methods, 65, 55 (1983).
Acknowledgment
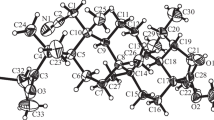
The work was financially supported by Russian Science Foundation grant No. 21-13-00161. We thank the Center for Collective Use of PFRC, UrB, RAS, Research on Materials and Compounds, for facilitating the spectral and analytical studies and Candidate M. V. Dmitriev, Department of Organic Chemistry, Perm State Natl. Res. Univ., for performing the XSA of 5b.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2023, pp. 71–76.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Krainova, G.F., Beloglazova, Y.A. & Grishko, V.V. Synthesis of 3-Methyl Derivatives from Dihydrobetulonic Acid Methyl Ester. Chem Nat Compd 59, 80–86 (2023). https://doi.org/10.1007/s10600-023-03923-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-023-03923-x