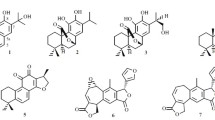
A new benzophenone derivative, hyperinagasone (1), has been isolated from the aerial part of Hypericum nagasawae together with six known compounds, trichadonic acid (2), norathyriol (3), euxanthone (4), subalatin (5), hyperielliptone D (6), and 1,4-O-diferuloylsecoisolariciresinol (7). The structure of new compound 1 was determined through spectroscopic and MS analyses. Among the isolates, compounds 2, 4, and 7 exhibited cytotoxicities, with IC50 values of 17.25 ± 1.55, 18.14 ± 1.07, and 10.31 ± 0.92 μM, respectively, against the DLD-1 cell line. Compounds 2, 5, and 7 also exhibited cytotoxicities, with IC50 values of 15.10 ± 1.13, 15.89 ± 0.94, and 17.54 ± 1.16 μM, respectively, against the CCRF-CEM cell line. Norathyriol (3) and 7 exhibited cytotoxicities, with IC50 values of 19.62 ± 2.88 and 6.26 ± 0.50 μM, respectively, against HL-60 cell line. Hyperinagasone (1) and 1,4-O-diferuloylsecoisolariciresinol (7) exhibited cytotoxicities, with IC50 values of 16.83 ± 1.55 and 11.38 ± 0.87 μM, respectively, against the IMR-32 cell line. In addition, 3, 4, and 7 showed potent inhibition, with IC50 values of 5.89 ± 0.43, 7.55 ± 0.54, and 8.12 ± 0.91 μM, respectively, against LPS-induced NO generation.
Similar content being viewed by others
References
N. K. B. Robson, Guttiferae in Flora of Taiwan, Vol. 2, 2nd Ed., Editorial Committee of the Flora of Taiwan, Taipei, Taiwan, 1996, pp. 694–714.
F. S. Wu, C. J. Hung, C. L. Lin, H. Y. Huang, Y. H. Kuo, T. H. Chang, C. L. Chen, P. J. Sung, M. J. Cheng, C. W. Kuo, and J. J. Chen, Chem. Nat. Compd., 57, 645 (2021).
D. Hong, F. Yin, L. H. Hu, and P. Lu, Phytochemistry, 65, 2595 (2004).
C. Y. Huang, T. C. Chang, Y. J. Wu, Y. Chen, and J. J. Chen, Molecules, 25, 4463 (2020).
Z. Y. Xiao, Y. H. Zeng, Q. Mu, W. K. P. Shiu, and S. Gibbons, Chem. Biodiv., 7, 953 (2010).
Q. Chen, L. Di, Y. Zhang, and N. Li, J. Ethnopharmacol., 259, 112948 (2020).
H. Zhu, C. Chen, J. Yang, X. N. Li, J. Liu, B. Sun, S. X. Huang, D. Li, G. Yao, Z. Luo, Y. Li, J. Zhang, Y. Xue, and Y. Zhang, Org. Lett., 16, 6322 (2014).
P. T. Nedialkov, D. Zheleva-Dimitrova, G. Momekov, K. Karlov, U. Girreser, and G. M. Kitanov, Nat. Prod. Res., 25, 1743 (2011).
M.-G. F. Guefack, F. Damen, A. T. Mbaveng, S. B. Tankeo, G. T. M. Bitchagno, I. Celik, J. D. S. Mpetga, and V. Kuete, Evid. Based Complement. Alternat. Med., 2020, 4314807 (2020).
C. Cirak, Biochem. Syst. Ecol., 34, 897 (2006).
T. T. Dao, T. T. Dang, P. H. Nguyen, E. Kim, P. T. Thuong, and W. K. Oh, Bioorg. Med. Chem. Lett., 22, 3688 (2012).
M.-T. Chen, Heterocycles, 27, 2589 (1988).
F. Damen, J. D. Simo Mpetga, O. M. F. Demgne, I. Celik, B. E. N. Wamba, L. A. Tapondjou, V. P. Beng, S. Levent, V. Kuete, M. Tene, Nat. Prod. Res., 36, 2071 (2022).
S.-S. Moon, A. A. Rahman, J.-Y. Kim, and S.-H. Kee, Bioorg. Med. Chem., 16, 7264 (2008).
T. Mosmann, J. Immunol. Meth., 65, 55 (1983).
M. Johansson, B. Kopcke, H. Anke, and O. Sterner, J. Antibiot., 55, 104 (2002).
Acknowledgment
This research was supported by the grant from the Ministry of Science and Technology, Taiwan (MOST 109-2320-B-010-029-MY3), awarded to Prof. J.-J. Chen. This work was also supported by the grants from Far Eastern Memorial Hospital–National Yang Ming Chiao Tung University Joint Research Program (110DN21), Medical Research Fund (KAFGH-A-110002) of Kaohsiung Armed Forces General Hospital, and Kaohsiung Veterans General Hospital Tainan Branch (VHYK110-07). We gratefully thank Ms. Shou-Ling Huang and Dr. Iren Wang for the assistance in NMR experiments of the Instrumentation Center at NTU, which is supported by the Ministry of Science and Technology, Taiwan. Fu-Sen Wu, I-Chou Wang, Chia-Ching Liaw, and Hsueh-Yang Huang have contributed equally to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 5, September–October, 2022, pp. 704–708.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, FS., Wang, IC., Liaw, CC. et al. New Benzophenone and Bioactive Constituents from Hypericum nagasawae. Chem Nat Compd 58, 833–838 (2022). https://doi.org/10.1007/s10600-022-03810-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-022-03810-x